Synthesis and Enzymatic Degradation of Sustainable Levoglucosenone-Derived Copolyesters with Renewable Citronellol Side Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
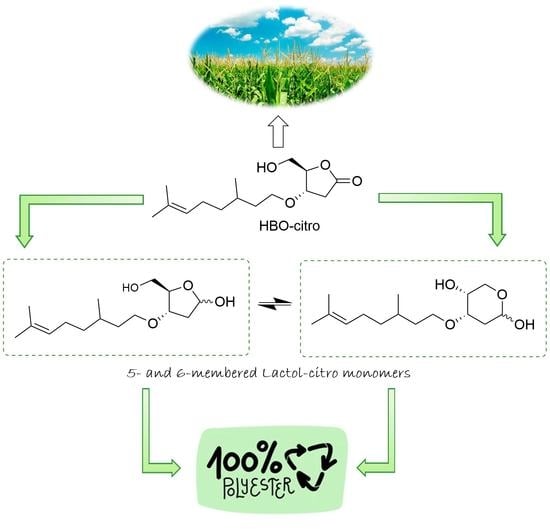
2.2. Synthesis of Monomers
2.3. Synthesis of Polymers
2.4. Enzymatic Degradation
3. Results and Discussion
3.1. Lactol-Citro
3.2. Polycondensation
3.3. Enzymatic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mecking, S. Nature or Petrochemistry?—Biologically Degradable Materials. Angew. Chem. Int. Ed. 2004, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, O.F.; Shishov, M.G.; Deryugin, A.A.; Sidelnikov, A.Y. Environmental Impact of Polymer-Waste Disposal. Coke Chem. 2016, 59, 117–121. [Google Scholar] [CrossRef]
- Sivan, A. New Perspectives in Plastic Biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers That Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Jyot Singh, J.; Sehgal, S. Biodegradation of Plastics: A State of the Art Review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable Polymers and Plastics: Performance beyond the Green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Höglund, A.; Odelius, K.; Albertsson, A.-C. Crucial Differences in the Hydrolytic Degradation between Industrial Polylactide and Laboratory-Scale Poly(L-Lactide). ACS Appl. Mater. Interfaces 2012, 4, 2788–2793. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, F.; Pan, Y.; Chen, C.; Jiang, L.; Dan, Y. Effect of Hydrophobic Fluoropolymer and Crystallinity on the Hydrolytic Degradation of Poly(Lactic Acid). Eur. Polym. J. 2017, 97, 308–318. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A Model for Hydrolytic Degradation and Erosion of Biodegradable Polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and Hydrolysis Rate of Aliphatic Aromatic Polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Brown, A.E.; Reinhart, K.A. Polyester Fiber: From Its Invention to Its Present Position. Science 1971, 173, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Deopura, B.L.; Padaki, N.V. Chapter 5—Synthetic Textile Fibres: Polyamide, Polyester and Aramid Fibres. In Textiles and Fashion; Sinclair, R., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2015; pp. 97–114. ISBN 978-1-84569-931-4. [Google Scholar]
- Rivard, C.; Moens, L.; Roberts, K.; Brigham, J.; Kelley, S. Starch Esters as Biodegradable Plastics: Effects of Ester Group Chain Length and Degree of Substitution on Anaerobic Biodegradation. Enzym. Microb. Technol. 1995, 17, 848–852. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lizundia, E. A Review on the Thermomechanical Properties and Biodegradation Behaviour of Polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable Polymers: Opportunities for the Next Decade. ACS Macro Lett. 2013, 2, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Fadlallah, S.; Sinha Roy, P.; Garnier, G.; Saito, K.; Allais, F. Are Lignin-Derived Monomers and Polymers Truly Sustainable? An in-Depth Green Metrics Calculations Approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Itawi, H.E.; Fadlallah, S.; Allais, F.; Perre, P. Green Assessment of Polymer Microparticles Production Processes: A Critical Review. Green Chem. 2022. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Antonietti, M.; Barner-Kowollik, C.; Binder, W.H.; Böker, A.; Boyer, C.; Buchmeiser, M.R.; Cheng, S.Z.D.; D’Agosto, F.; Floudas, G.; et al. The Next 100 Years of Polymer Science. Macromol. Chem. Phys. 2020, 221, 2000216. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.-A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef]
- Seppälä, J.; van Bochove, B.; Lendlein, A. Developing Advanced Functional Polymers for Biomedical Applications. Biomacromolecules 2020, 21, 273–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. Citronellol, a Monoterpene Alcohol with Promising Pharmacological Activities—A Systematic Review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.L.; Morozowich, N.L.; Decker, T.E.; Allcock, H.R. Crosslinkable Citronellol Containing Polyphosphazenes and Their Biomedical Potential. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2258–2265. [Google Scholar] [CrossRef]
- Nichol, J.L.; Allcock, H.R. Polyphosphazenes with Amino Acid Citronellol Ester Side Groups for Biomedical Applications. Eur. Polym. J. 2015, 62, 214–221. [Google Scholar] [CrossRef]
- Singler, R.E.; Schneider, N.S.; Hagnauer, G.L. Polyphosphazenes: Synthesis—Properties—Applications. Polym. Eng. Sci. 1975, 15, 321–338. [Google Scholar] [CrossRef]
- Worzakowska, M. High Chemical and Solvent Resistant, Branched Terpene Methacrylate Polymers—Preparation, Thermal Properties, and Decomposition Mechanism. Polym. Adv. Technol. 2018, 29, 1414–1425. [Google Scholar] [CrossRef]
- Ariel, M.S.; Maria, M.Z.; Rolando, A.S. Recent Applications of Levoglucosenone as Chiral Synthon. Curr. Org. Synth. 2012, 9, 439–459. [Google Scholar]
- Clark, J.H.; Bruyn, M.D.; Budarin, V.L. Method for Producing Levoglucosenone. U.S. Patent 10,774,089, 15 September 2016. [Google Scholar]
- Comba, M.B.; Tsai, Y.; Sarotti, A.M.; Mangione, M.I.; Suárez, A.G.; Spanevello, R.A. Levoglucosenone and Its New Applications: Valorization of Cellulose Residues. Eur. J. Org. Chem. 2018, 2018, 590–604. [Google Scholar] [CrossRef]
- Fadlallah, S.; Mouterde, L.M.M.; Garnier, G.; Saito, K.; Allais, F. Cellulose-Derived Levoglucosenone, a Great Versatile Chemical Platform for the Production of Renewable Monomers and Polymers. In Sustainability & Green Polymer Chemistry Volume 2: Biocatalysis and Biobased Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1373, pp. 77–97. ISBN 978-0-8412-9852-1. [Google Scholar]
- Fadlallah, S.; Peru, A.A.M.; Longé, L.; Allais, F. Chemo-Enzymatic Synthesis of a Levoglucosenone-Derived Bi-Functional Monomer and Its Ring-Opening Metathesis Polymerization in the Green Solvent CyreneTM. Polym. Chem. 2020, 11, 7471–7475. [Google Scholar] [CrossRef]
- Fadlallah, S.; Peru, A.A.M.; Flourat, A.L.; Allais, F. A Straightforward Access to Functionalizable Polymers through Ring-Opening Metathesis Polymerization of Levoglucosenone-Derived Monomers. Eur. Polym. J. 2020, 138, 109980. [Google Scholar] [CrossRef]
- Kayishaer, A.; Fadlallah, S.; Mouterde, L.M.M.; Peru, A.A.M.; Werghi, Y.; Brunois, F.; Carboué, Q.; Lopez, M.; Allais, F. Unprecedented Biodegradable Cellulose-Derived Polyesters with Pendant Citronellol Moieties: From Monomer Synthesis to Enzymatic Degradation. Molecules 2021, 26, 7672. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, S.; Kayishaer, A.; Annatelli, M.; Mouterde, L.M.M.; Peru, A.A.M.; Aricò, F.; Allais, F. Fully Renewable Photocrosslinkable Polycarbonates from Cellulose-Derived Monomers. Green Chem. 2022, 24, 2871–2881. [Google Scholar] [CrossRef]
- Diot-Néant, F.; Mouterde, L.; Fadlallah, S.; Miller, S.A.; Allais, F. Sustainable Synthesis and Polycondensation of Levoglucosenone-Cyrene-Based Bicyclic Diol Monomer: Access to Renewable Polyesters. ChemSusChem 2020, 13, 2613–2620. [Google Scholar] [CrossRef]
- Tsai, Y.; Borini Etichetti, C.M.; Cicetti, S.; Girardini, J.E.; Spanevello, R.A.; Suárez, A.G.; Sarotti, A.M. Design, Synthesis and Evaluation of Novel Levoglucosenone Derivatives as Promising Anticancer Agents. Bioorganic Med. Chem. Lett. 2020, 30, 127247. [Google Scholar] [CrossRef] [PubMed]
- Mackie, W.; Perlin, A.S. Pyranose–Furanose and Anomeric Equilibria: Influence of Solvent and of Partial Methylation. Can. J. Chem. 1966, 44, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Alejandra, R.-C.; Margarita, C.-M.; María Soledad, M.-C. Enzymatic Degradation of Poly(3-Hydroxybutyrate) by a Commercial Lipase. Polym. Degrad. Stab. 2012, 97, 2473–2476. [Google Scholar] [CrossRef]









 | |||||
|---|---|---|---|---|---|
| Run | Polymer | Mn (kDa) 1 | Đ1 | Tg (°C) 2 | Td50% (°C) 3 |
| 1 | P5 | - 4 | - | −62 | 198 |
| 2 | P6 | - 4 | - | −57 | 205 |
| 3 | P7 | 12.5 | 1.3 | −67 | 243 |
| 4 | P8 | 25.8 | 1.8 | - | 210 |
| Run 1 | Polymer | Mn (kDa) 2 | Tg (°C) 3 | ΔTg (°C) 4 | 1H NMR 5 | FTIR 6 |
|---|---|---|---|---|---|---|
| 1 | P5 | 0.7 | 46 | 108 | ✓ | ✓ |
| 2 | P6 | 0.8 | 55 | 112 | ✓ | ✓ |
| 3 | P7 | 0.8 | 44 | 111 | ✓ | ✓ |
| 4 | P8 | 0.8 | 74 | - 7 | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadlallah, S.; Carboué, Q.; Mouterde, L.M.M.; Kayishaer, A.; Werghi, Y.; Peru, A.A.M.; Lopez, M.; Allais, F. Synthesis and Enzymatic Degradation of Sustainable Levoglucosenone-Derived Copolyesters with Renewable Citronellol Side Chains. Polymers 2022, 14, 2082. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102082
Fadlallah S, Carboué Q, Mouterde LMM, Kayishaer A, Werghi Y, Peru AAM, Lopez M, Allais F. Synthesis and Enzymatic Degradation of Sustainable Levoglucosenone-Derived Copolyesters with Renewable Citronellol Side Chains. Polymers. 2022; 14(10):2082. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102082
Chicago/Turabian StyleFadlallah, Sami, Quentin Carboué, Louis M. M. Mouterde, Aihemaiti Kayishaer, Yasmine Werghi, Aurélien A. M. Peru, Michel Lopez, and Florent Allais. 2022. "Synthesis and Enzymatic Degradation of Sustainable Levoglucosenone-Derived Copolyesters with Renewable Citronellol Side Chains" Polymers 14, no. 10: 2082. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102082