Modification of a Phenolic Resin with Epoxy- and Methacrylate-Functionalized Silica Sols to Improve the Ablation Resistance of Their Glass Fiber-Reinforced Composites
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
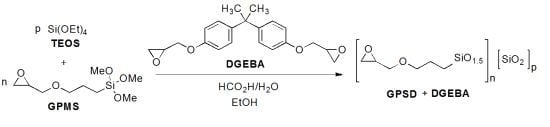
2.2. Preparation of Composites
2.3. Characterization and Measurements
3. Results and Discussion
3.1. Ablation Tests
| Matrix | Linear ablation (mm·s−1) | Mass ablation (g·s−1) |
|---|---|---|
| GPSD-PR | 0.230 | 0.117 |
| MPS-PR | 0.268 | 0.125 |
| GPS-MPS-PR | 0.279 | 0.140 |
| PR | 0.316 | 0.405 |
3.2. Morphologies of Ablation Structures

3.3. Temperature Evolution during Ablation Tests

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rosensweig, R.E.; Beecher, N. Theory for the ablation of fiberglass-reinforced phenolic resin. AIAA J. 1963, 1, 1802–1809. [Google Scholar] [CrossRef]
- Geier, M. Space refractory materials. Metaux Corros. Idustrie 1969, 523, 92–128. [Google Scholar]
- Koo, J.H.; Stretz, H.; Weispfenning, J.T.; Luo, Z.; Wootan, W. Nanocomposite Rocket Ablative Materials: Processing, Microstructure and Performance. In Proceedings of 45th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics & Materials Conference, Palm Springs, CA, USA, 19–22 April 2004.
- Koo, J.H.; Pilato, L.A.; Wissler, G.E. Polymer nanostructured materials for propulsion systems. J. Spacecr. Rockets 2007, 44, 1250–1262. [Google Scholar] [CrossRef]
- Li, W.; Liu, F.; Wei, L.; Zhao, T. Synthesis, morphology and properties of polydimethylsiloxane-modified allylated novolac/4,4′-bismaleimidodiphenylmethane. Eur. Polym. J. 2006, 42, 580–592. [Google Scholar] [CrossRef]
- Hung, A.Y.C.; Wang, F.Y.; Yeh, S.R.; Chen, W.J.M.; Ma, C.C. Carbon/carbon composites derived from poly(ethylene oxide)-modified novolac-type phenolic resin: Microstructure, physical and morphological properties. J. Appl. Polym. Sci. 2002, 84, 1609–1619. [Google Scholar] [CrossRef]
- Gloria, A.; Ronca, D.; Russo, T.; D’Amora, U.; Chierchia, M.; de Santis, R.; Nicolais, L.; Ambrosio, L. Technical features and criteria in designing fiber-reinforced composite materials: from the aerospace and aeronautical field to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 151–163. [Google Scholar]
- Winya, N.; Boonpan, A.; Prapunkarn, K. Study of factors affecting the ablation rate of phenolic resin/fiber glass. Int. J. Chem. Eng. Appl. 2013, 4, 234–237. [Google Scholar]
- Qiu, J.; Cao, X.; Tian, C.; Zhang, J. Ablation performance of a novel super-hybrid composite. J. Mater. Sci. Technol. 2005, 21, 269–273. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Sun, D. Nanoindentation and nanoscratch profiles of hybrid films based on (γ-methacrylpropyl)trimethoxysilane and tetraethoxysilane. Acta Mater. 2006, 54, 5469–5475. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Chiang, C.L.; Ma, C.C.M.; Wu, D.L.; Kuan, H.C. Preparation, characterization, and properties of novolac-type phenolic/SiO2 hybrid organic–inorganic nanocomposite materials by sol-gel method. J. Appl. Polym. Sci. A 2003, 41, 905–913. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Sun, D.; Zhao, W. Three-dimensional configurations of organic/inorganic hybrid nanostructureal blocks: A quantum chemical investigation for cage structure of (γ-glycidyloxy)propylsilsesquioxane. J. Mol. Struct. 2008, 872, 197–204. [Google Scholar] [CrossRef]
- Chen, P.; Hu, L.; Zhang, X.; Sun, D. Enhanced corrosion resistance for silsesquioxane coatings by diglycidyl ether of bisphenol A. Mater. Sci. Pol. 2007, 25, 843–849. [Google Scholar]
- Wu, K.; Song, L.; Hu, Y.; Lu, H.; Kandola, B.K.; Kandare, E. Synthesis and characterization of a functional polyhedral oligomeric silsesquioxane and its flame retardancy in epoxy resin. Prog. Org. Coat. 2009, 65, 490–497. [Google Scholar] [CrossRef]
- Fasce, D.P.; Williams, R.J.J.; Erra-Balsells, R.; Ishikawa, Y.; Nonami, H. One-step synthesis of polyhedral silsesquioxanes bearing bulky substituents: UV-MALDI-TOF and ESI-TOF mass spectrometry characterization of reaction products. Macromolecules 2001, 34, 3534–3539. [Google Scholar] [CrossRef]
- Williams, R.J.J.; Erra-Balsells, R.; Ishikawa, Y.; Nonami, H.; Mauri, A.N.; Riccardi, C.C. UV-MALDI-TOF and ESI-TOF mass spectrometry characterization of silsesquioxanes obtained by the hydrolytic condensation of (γ-glycidoxypropyl)trimethoxysilane in an epoxidized solvent. Macromol. Chem. Phys. 2001, 202, 2425–2433. [Google Scholar] [CrossRef]
- Eisenberg, P.; Erra-Balsells, R.; Ishikawa, Y.; Lucas, J.C.; Nonami, H.; Williams, R.J.J. Silsesquioxanes derived from the bulk polycondensation of [γ-(methacryloxy)propyl] trimethoxysilane with concentrated formic acid: Evolution of molar mass distributions and fraction of intramolecular cycles. Macromolecules 2002, 35, 1160–1174. [Google Scholar]
- Liu, Y.; Wang, D.; Hu, L.; Liu, J. Study on Phenolic-Resin/Carbon-fiber Ablation Composites Modified with Polyhedral Oligomeric Silsesquioxanes. In Proceedings of the 4th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 605–608.
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002; pp. 24–29. [Google Scholar]
- Smith, M.E.; Ishida, H. Kinetics of the condensation reaction of epoxide with phenol: Linear chain growth versus branching. Macromolecules 1994, 27, 2701–2707. [Google Scholar] [CrossRef]
- Al Dwayyan, A.S.; Khan, M.N.; Salhi, S.A. Optical characterization of chemically etched nanoporous silicon embedded in sol-gel matrix. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Loy, D.A.; Baugher, B.M.; Baugher, C.R.; Schneider, D.A.; Rahimian, K. Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem. Mater. 2000, 12, 3624–3632. [Google Scholar] [CrossRef]
- Joseph, P.; Tretsiakova-Mcnally, S. Reactive modifications of some chain-and step-growth polymers with phosphorus-containing compounds: Effects on flame retardance-a review. Polym. Adv. Technol. 2011, 22, 395–406. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, Y.; Geng, W.; You, H.; Wang, Y.; Loy, D.A. Modification of a Phenolic Resin with Epoxy- and Methacrylate-Functionalized Silica Sols to Improve the Ablation Resistance of Their Glass Fiber-Reinforced Composites. Polymers 2014, 6, 105-113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6010105
Hu Y, Geng W, You H, Wang Y, Loy DA. Modification of a Phenolic Resin with Epoxy- and Methacrylate-Functionalized Silica Sols to Improve the Ablation Resistance of Their Glass Fiber-Reinforced Composites. Polymers. 2014; 6(1):105-113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6010105
Chicago/Turabian StyleHu, Yu, Wenlong Geng, Hong You, You Wang, and Douglas A. Loy. 2014. "Modification of a Phenolic Resin with Epoxy- and Methacrylate-Functionalized Silica Sols to Improve the Ablation Resistance of Their Glass Fiber-Reinforced Composites" Polymers 6, no. 1: 105-113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6010105