3.1. Optimization of Hydrogel Formulation
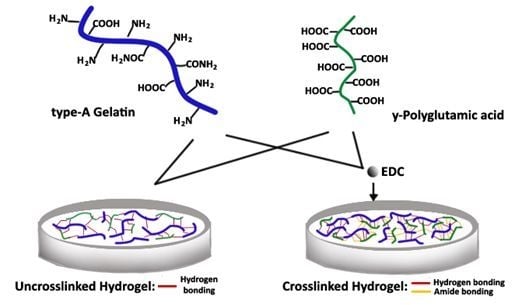
The hydrogel was prepared by the ionic interaction of type-A gelatin and γ-PGA with EDC as the crosslinker. During synthesis at pH = 7.4, gelatin with an isoelectric point (IEP) of 9.0 is cationic, while γ-PGA is naturally anionic. In
Figure 1, the interaction between the polymers is illustrated. The type-A gelatin charge is mainly due to its carboxyl, amino and guanidino groups on the side chains [
24,
25]. On the γ-PGA side chain, only the carboxyl group is present [
26]. Using the EDC, gelation of the two polymers at a lower temperature triggers the formation of an amide bond between the carboxyl group of γ-PGA and the amino group of gelatin. A hydrogen bond is also present between the two polymers. By varying the concentration of the two polymers and the crosslinker, the crosslinking density changes, which is represented by the change in its water uptake as SD (swelling degree) [
27].
For the first 2 h of the swelling kinetics of increasing γ-PGA, as shown in
Figure 2a, the means of four different formulations have no statistical difference. Notably, the SD of 1% γ-PGA has the lowest value. After 4 h of swelling, 4% γ-PGA with an SD of 19.01 ±0.18 is significantly different from 1% γ-PGA with an SD 16.86 ± 0.60. At the last swelling time of 6 h, 4% γ-PGA posted the highest SD of 20.58 ± 0.35, which is an 8% increase from its SD at 4 h and an 18% increase from the initial SD at 2 h. Among all formulations, only 0.5% and 1% γ-PGA SD did not significantly change during the 6-h swelling.
Notably, the trend is that as the concentration of γ-PGA increases, the SD also increases, but this is not evident between 0.5% and 1%, wherein it decreases. This only proves that the optimal concentration of the carboxyl group from γ-PGA to interact for hydrogel formation is 1%, above which, excess γ-PGA will interfere with the gelation process [
28]. Although, the SD means of 0.5% and 1% are not statistically different from the entire 6 h of swelling.
Figure 1.
The effect of the crosslinker [ethylcarbodiimide (EDC)] on the ionic interaction of type-A gelatin and γ-Polyglutamic acid (γ-PGA) for the synthesis of hydrogel at a pH=7.4 and a temperature of 4 °C.
Figure 1.
The effect of the crosslinker [ethylcarbodiimide (EDC)] on the ionic interaction of type-A gelatin and γ-Polyglutamic acid (γ-PGA) for the synthesis of hydrogel at a pH=7.4 and a temperature of 4 °C.
Figure 2.
(a) The effect of increasing concentration of γ-PGA [0.5, one, two and 4 w/v% (g/mL)] on the swelling degree (SD) of hydrogel at a constant formulation of 10 w/v% gelatin and of 1.3 mg/mL EDC, swelled in phosphate buffered saline (PBS) (pH = 7.4) for 6 h; (b) The effect of increasing concentration of gelatin [eight, 10 and 12 w/v% (g/mL)] on the swelling degree (SD) of hydrogel at a constant formulation of 1 w/v% γ-PGA and of 1.3 mg/mL EDC, swelled in PBS (pH = 7.4) for 6 h.
Figure 2.
(a) The effect of increasing concentration of γ-PGA [0.5, one, two and 4 w/v% (g/mL)] on the swelling degree (SD) of hydrogel at a constant formulation of 10 w/v% gelatin and of 1.3 mg/mL EDC, swelled in phosphate buffered saline (PBS) (pH = 7.4) for 6 h; (b) The effect of increasing concentration of gelatin [eight, 10 and 12 w/v% (g/mL)] on the swelling degree (SD) of hydrogel at a constant formulation of 1 w/v% γ-PGA and of 1.3 mg/mL EDC, swelled in PBS (pH = 7.4) for 6 h.
Conversely, there is an inverse relationship between increasing concentration of gelatin and its SD, as shown in
Figure 2b. Hydrogel of 8% gelatin, swelled for six hours, has the highest value of SD, 20.45 ± 0.31, but there is no significant difference in its SD in the entire swelling time. Similarly, the SD of 10% gelatin did not significantly increase from 2 to 6 h of swelling. On the other hand, the SD of 12% gelatin increases significantly by about 6%, from an SD of 15.05 ± 0.04 after 2 h of swelling to 16.01 ± 0.19 for 6 h of swelling.
The chemical crosslinker used is EDC (carbodiimide) is a non-toxic water soluble compound that acts as a catalyst for the formation of the amide bond between the amino groups of lysine and hydroxylysine of gelatin to the carboxylic groups of γ-PGA or to the carboxylic groups of glutamic and aspartic acid of another gelatin strand. EDC is not integrated in the linkage, as it only facilitates the formation of the bond, making it a suitable crosslinker [
25].
As shown in
Figure 3a, the SD of uncrosslinked hydrogel of a value of 19.28 ± 0.27 has a 13% difference compared to crosslinked hydrogel with 0.75 mg/mL EDC of a value of 17.04 ± 0.67 after 2 h of swelling. This represents the increase of its crosslinking density. Hydrogel with 1.3 mg/mL EDC has the only significant change throughout the swelling time of 2–6 h, in comparison to 0.75 and 2.5 mg/mL EDC and uncrosslinked hydrogel. Thus, the ideal amount of EDC to be used in synthesizing hydrogel is 1.3 mg/mL EDC.
The swelling degree is one of the considerations in deciding the ideal formulation for synthesizing hydrogel; since, as indicated in various studies, SD has a proportional relationship with drug release [
29]. In
Figure 3b, the plot of SD against the ratio of the concentrations of the two polymers proved that there is an optimal value of γ-PGA for a specific amount of gelatin manifested by different SD properties. Theoretically, during drug release, the swelling of hydrogel will take place first, and then, diffusion of the drug from the hydrogel to the solution will follow. Hence, when controlled release is desired, a low value of SD with only a gradual increase against time is preferable. Hydrogel synthesized with 10 w/v% gelatin and 1 w/v% γ-PGA showed a gradual increase in SD, making it the optimal formulation. Although, the formulation with 12% gelatin and 1% γ-PGA has the lowest SD, its SD value greatly increases with time, which can have a negative result on drug release.
Figure 3.
(a) The effect of increasing concentration of EDC (zero, 0.75, 1.3 and 2.5 mg/mL) to the swelling degree (SD) of hydrogel at a constant gelatin concentration of 10 w/v% and γ-PGA of 1 w/v%, swelled in PBS (pH=7.4) for 6 h; (b) The swelling degree of hydrogel with a constant EDC of 1.3 mg/mL in PBS (pH = 7.4) of different ratios of gelatin (eight, 10 and 12 w/v%) over -PGA (0.5, one, two and 4 w/v%) (-○-, 2 h; -□-, 4 h; -∆-, 6 h of swelling).
Figure 3.
(a) The effect of increasing concentration of EDC (zero, 0.75, 1.3 and 2.5 mg/mL) to the swelling degree (SD) of hydrogel at a constant gelatin concentration of 10 w/v% and γ-PGA of 1 w/v%, swelled in PBS (pH=7.4) for 6 h; (b) The swelling degree of hydrogel with a constant EDC of 1.3 mg/mL in PBS (pH = 7.4) of different ratios of gelatin (eight, 10 and 12 w/v%) over -PGA (0.5, one, two and 4 w/v%) (-○-, 2 h; -□-, 4 h; -∆-, 6 h of swelling).
3.2. Characterization of Gelatin-γ-PGA-Based Hydrogel
The FT-IR spectra of the two polymers and EGCG are shown in
Figure 4. The characteristic peak at 1643 cm
−1 in the γ-PGA spectrum represents carboxylic ions (–COO
−), while in the gelatin spectrum, the observed peak at 1535 cm
−1 is for a protonated amino group (–NH
3+) or N–H (amide) [
30,
31]. The characteristic peak assigned to the aliphatic side chain of gelatin is at 2970 cm
−1 [
32]. Comparing it with the peaks of freeze-dried crosslinked unloaded hydrogel, the 1643 cm
−1 peak for γ-PGA shifted to 1650 cm
−1, and the peak 1535 cm
−1 for gelatin moved to 1542.8 cm
−1. This proves the ionic interaction between the negatively charged carboxylic group (–COO
−) of γ-PGA and the positively charged amino group (–NH
3+) of gelatin, forming the insoluble hydrogel. As studied previously, a shift at peaks of interacting groups of different polymers indicates the formed hydrogen bond or other types of bonds between the chemical or functional groups [
33].
The characteristic bands in the FT-IR spectrum of extracted EGCG (65% purity) at 822, 1030, 1146, 1234, 1342, 1458, 1523 and 1620 cm
−1 indicate the presence of C–H alkenes, –C–O alcohols, C–OH alcohols, –OH aromatic, C–O alcohols, C-H alkanes, C=C aromatic ring and C=C alkenes, respectively. At a wavenumber higher than 3200 cm
−1, such as the characteristic band with a peak at 3363 corresponds to O-H linkage of phenolic and hydroxyl groups [
34]. The characteristic band of EGCG at 1,234 cm
−1 indicates that the -OH aromatic is retained in the spectrum of EGCG-loaded hydrogel. The 1030 cm
−1 band for EGCG shifted to 1038 cm
−1, from 1146 to 1153 cm
−1 and from 1342 to 1338 cm
−1, respectively, after loading it in the hydrogel. The peaks of unloaded hydrogel at 1080, 1454, 1543 and 1651 cm
−1 shifted to 1088, 1450, 1531 and 1655 cm
−1, respectively. These peak shifts only suggest that EGCG is effectively loaded to the hydrogel. Furthermore, the characteristic peak of EGCG at 3363 shifted to a broad peak at 3390 cm
−1, indicating the interaction of EGCG with gelatin and γ-PGA when loaded.
Figure 4.
FT-IR spectra of extracted epigallocatechin gallate (EGCG) (65% purity), type-A gelatin, γ-PGA, freeze-dried, unloaded hydrogel (10%-gelatin, 1%-γ-PGA and 1.3 mg/mL EDC) and EGCG-loaded hydrogel of the same formulation as that of the unloaded one. Peaks in the gelatin spectrum are not clearly shown, as its percentage of transmittance is relatively small.
Figure 4.
FT-IR spectra of extracted epigallocatechin gallate (EGCG) (65% purity), type-A gelatin, γ-PGA, freeze-dried, unloaded hydrogel (10%-gelatin, 1%-γ-PGA and 1.3 mg/mL EDC) and EGCG-loaded hydrogel of the same formulation as that of the unloaded one. Peaks in the gelatin spectrum are not clearly shown, as its percentage of transmittance is relatively small.
Thermal stability of hydrogels, as well as miscibility between gelatin and γ-PGA are studied using DSC. Thermograms are presented in
Figure 5, where the effect of the crosslinker on the thermal properties of the hydrogel is also determined.
Figure 5.
Thermogram of gelatin, γ-PGA, uncrosslinked (no EDC) and crosslinked hydrogels using 0.75, 1.3 and 2.5 mg/mL EDC at a heating rate of 10 °C/min. Tm, melting point; Tg, glass transition temperature.
Figure 5.
Thermogram of gelatin, γ-PGA, uncrosslinked (no EDC) and crosslinked hydrogels using 0.75, 1.3 and 2.5 mg/mL EDC at a heating rate of 10 °C/min. Tm, melting point; Tg, glass transition temperature.
The thermogram of γ-PGA only shows one peak at approximately 73 °C, which is associated with its melting point, Tm. The thermal properties of hydrogel are affected by water content, and since hydrogel is hygroscopic, preparation upon DSC analysis can alter its water content and change its thermal property in comparison to what is usually reported in the literature [
35]. In the gelatin thermogram, a broad peak at 82 °C and a relatively small peak at 225 °C correspond to its glass transition temperature, Tg, and Tm, respectively [
36]. The uncrosslinked hydrogel thermogram shows a peak at 87 °C, relatively different from the Tg and Tm of gelatin and γ-PGA. Another peak is observed at 212 °C, which is lower than the Tm of gelatin. The shifts in the endothermic peaks of hydrogel in comparison to the peaks of its polymers suggested the interaction and miscibility of gelatin and γ-PGA having new distinct thermal properties. Crosslinking the hydrogel with 0.75 mg/mL EDC did not change the thermal properties of the hydrogel significantly with its Tg of 87 °C and Tm of 211 °C. However, by using 1.3 mg/mL EDC, there is a change in its Tg, having a new peak at 80 °C, but the same Tm of 212 °C. Lastly, at the highest concentration of EDC at 2.5 mg/mL, the Tg is at 80 °C and the Tm changes to 209 °C.
The surface morphology and polymer networks of hydrogels are studied using SEM, as shown in
Figure 6 for the surface and
Figure 7 for the cross-section. Crosslinking of hydrogel did not change the surface of the freeze-dried hydrogel, as shown in
Figure 6b–d, compared to
Figure 6a. This result is consistent with having a rough wrinkled surface, but without any holes. In the network of hydrogel inside, there is a change, due to crosslinking, as illustrated by
Figure 7a, compared to
Figure 7b–d. As the crosslinking concentration increases, the network becomes more organized, and the pores become smaller. The void spaces are the result of the freeze-drying of the synthesized hydrogel. These structures suggest that the mechanism during swelling is for the solvent to diffuse first into the surface layer before the void spaces between networks of hydrogel are filled up.
After loading of EGCG and a second freeze-drying, the surface of the hydrogel becomes smooth (
Figure 6e,f) and the network becomes more systematic (
Figure 7e), with a pore size of approximately 50 μm (
Figure 7f).
Figure 6.
Scanning electron micrograph of the surface of freeze-dried hydrogel at 300× for (a–e) and 1000× for (f): (a) uncrosslinked; (b) with 0.75 mg/mL EDC; (c) with 1.3 mg/mL EDC; (d) with 2.5 mg/mL EDC; (e,f) EGCG-loaded hydrogel.
Figure 6.
Scanning electron micrograph of the surface of freeze-dried hydrogel at 300× for (a–e) and 1000× for (f): (a) uncrosslinked; (b) with 0.75 mg/mL EDC; (c) with 1.3 mg/mL EDC; (d) with 2.5 mg/mL EDC; (e,f) EGCG-loaded hydrogel.
Figure 7.
Scanning electron micrograph of the cross-section of freeze-dried hydrogel at 300× for (a–e) and 1000× for (f): (a) uncrosslinked; (b) with 0.75 mg/mL EDC; (c) with 1.3 mg/mL EDC; (d) with 2.5 mg/mL EDC; (e,f) EGCG-loaded hydrogel. The scale bar = 10 μm.
Figure 7.
Scanning electron micrograph of the cross-section of freeze-dried hydrogel at 300× for (a–e) and 1000× for (f): (a) uncrosslinked; (b) with 0.75 mg/mL EDC; (c) with 1.3 mg/mL EDC; (d) with 2.5 mg/mL EDC; (e,f) EGCG-loaded hydrogel. The scale bar = 10 μm.
3.3. pH-Responsiveness of Hydrogel
Hydrogels composed of polymers with ionic groups or dependent on ionic interaction between polymers respond to external pH. As the ionic group is either protonated or deprotonated, depending on its nature and pKa, the SD of the hydrogel significantly changes with pH [
37,
38].
The pH responsiveness of gelatin-γ-PGA-based hydrogel was determined with a phosphate buffer solution of pH = 4.5 and 9.0, since these values approach the pKa and IEP of glutamic acid and gelatin (high boom), respectively [
17,
25]. The pH values of 6.8 and 7.4 were also chosen, because these were used in
in vitro release studies.
The SD at different pH values of uncrosslinked hydrogel (
Figure 8a) is not statistically different from 2 h up to 4 h of swelling time. However, in the sixth hour of swelling, the SD at pH 9.0 is significantly different from the SD at pH 4.5 and pH 6.8. In other words, uncrosslinked hydrogel is more sensitive to higher pH approaching the pKa of gelatin. Hence, some of the positively charged amino groups of gelatin are being deprotonated. As a result, repulsion among a negatively charged carboxylic group of γ-PGA and gelatin occurs, increasing the SD, as has also been reported in other studies using γ-PGA [
15]. In comparison to pH 4.5, there is still an interaction, not in terms of gelatin and γ-PGA, but between carboxylic group of gelatin and an amino group of another gelatin strand, as evidenced by the near constant value of the SD at pH 4.5 throughout 6 h of swelling.
Figure 8b clearly shows that the SD at pH 7.4 is statistically different from the SD at pH 4.5, 6.8 and 9.0. The properties of crosslinked hydrogel differ from uncrosslinked hydrogel, because of the presence of an amide bond formed (
Figure 1). Meanwhile, the hydrogen bond is present in the hydrogel, regardless of whether using a crosslinker. At neutral pH 7.4, the amide bond, as well as the hydrogen bond are strong, making a compact and tight structure. Thus, significant movement of the network upon the absorption of water is not allowed. This results in a lower SD with a value of only 17.72 ± 0.02 after 6 h of swelling. Therefore, the presence of an amide bond has a big impact on the SD of crosslinked hydrogel at different pH values.
Figure 8.
(a) Swelling kinetics of uncrosslinked hydrogel (10 w/v% gelatin and 1 w/v% γ-PGA) at different pH values for two, four and 6 h. (b) Swelling kinetics of optimized formulation of crosslinked hydrogel (10 w/v% gelatin, 1 w/v% γ-PGA and 1.3 mg/mL EDC) at different pH values for two, four and 6 h.
Figure 8.
(a) Swelling kinetics of uncrosslinked hydrogel (10 w/v% gelatin and 1 w/v% γ-PGA) at different pH values for two, four and 6 h. (b) Swelling kinetics of optimized formulation of crosslinked hydrogel (10 w/v% gelatin, 1 w/v% γ-PGA and 1.3 mg/mL EDC) at different pH values for two, four and 6 h.
3.4. Loading and In Vitro Release Kinetics Study
EGCG was loaded into hydrogel by the post-loading method, since in situ loading is not possible, as EGCG is sensitive to oxidation. Then, freeze-drying is used to remove the solvent absorbed during loading. The optimized loading time is determined to be 12 h. A loading time longer than 12 h will not result in a significant increase in the loading efficiency, but will only expose the EGCG to further degradation and oxidation.
As the crosslinking concentration increases, the hydrogel loading efficiency decreases, as shown in
Table 1. This inverse relationship is related to its decreasing SD as EDC concentration increases (
Figure 3a); whereas the percent difference between the SD of hydrogel with 0.75 and 1.3 mg/mL EDC at 6 h of swelling is 1.21%, similar to the percent difference in their loading efficiency of 1.97%, also the percent difference between the SD at 1.3 and 2.5 mg/mL EDC of 7.55% is similar to the difference in their loading efficiency of 7.16%. This suggests that the loading efficiency of hydrogel is dependent on its SD. It can be interpreted that the diffusion of EGCG into the hydrogel with 2.5 mg/mL EDC is limited, due to a low SD. The mechanism of loading EGCG is comprised of the swelling of hydrogel, followed by the diffusion of EGCG into the surface, up to the networks of the hydrogel inside. Then, during freeze-drying, only absorbed water is removed from the hydrogel. Consequently, the difference between the loading efficiency of hydrogel with 0.75 and 1.3 mg/mL EDC is relatively small compared to 2.5 mg/mL. Although, 2.5 mg/mL posted the lowest SD, which is the ideal property to retard the release of drug, it has also the lowest loading efficiency. The optimal EDC concentration is 1.3 mg/mL in terms of loading efficiency and similar when the basis is in terms of swelling degree (
Figure 3a).
Table 1.
The effect of the crosslinker on the loading efficiency of hydrogel
Table 1.
The effect of the crosslinker on the loading efficiency of hydrogel
| Gelatin concentration (w/v%) | γ-PGA concentration (w/v%) | EDC concentration (mg/mL) | Loading efficiency (%) |
|---|
| 10 | 1 | 0.75 | 68.71 ± 1.47 |
| 1.3 | 67.37 ± 0.43 |
| 2.5 | 62.72 ± 2.13 |
The
in vitro release kinetics of EGCG from hydrogel was studied at a temperature of 37 °C using HCl solution at pH 1.2 and PBS (0.15 M) at pH 6.8 and 7.4. The pH of GIT changes and is always varying, but these three pH values have been used in many studies conducting preliminary and
in vitro release tests to mimic the pH conditions of the GIT. A solution at pH 1.2 represents gastric fluid in the stomach, while buffer solutions at pH 6.8 and pH 7.4 simulate the jejunal region of small intestine and the ileum segment, respectively [
20,
23,
39].
The optimized formulation of hydrogel at pH 1.2 posted a 51.72% cumulative release of EGCG compared to a lower EGCG release at pH 6.8 and 7.4 after 12 h (
Figure 9a). In contrast, uncrosslinked hydrogel (
Figure 9b) shows a high rate of EGCG release, with 85.07% cumulative release in only 5 h at pH 7.4. The release profile using uncrosslinked hydrogel was only monitored up to 5 h at pH 7.4 and 1.5 h at pH 6.8. This is because, past these hours, the release medium becomes cloudy, due to the disintegration of the hydrogel. A cloudy aliquot significantly affects the absorbance of the medium, causing errors in the UV determination of the released EGCG.
The crosslinking of hydrogel can greatly affect its release profile properties (
Figure 9a,b) and pH responsiveness (
Figure 8a,b). Notably, the release profile of EGCG at any pH using the optimized crosslinked hydrogel shows no observed burst release. This good property of the synthesized hydrogel suggests a sustained release of EGCG that can last up to several hours and withstand an environment of low to neutral pH. In the GIT, the hydrogel is first exposed to a low pH of 1.2 for about 2 h, the average gastric emptying time [
39]. Based on these conditions, this accounts for about 21.97% of EGCG released, after which, there is still a significant amount of EGCG remaining in the hydrogel to be released in the succeeding path of GIT.
Figure 9.
(a) In vitro release kinetics of EGCG from the optimized formulation of crosslinked hydrogel in PBS of different pH values (-○-, pH 1.2; -□-, pH 6.8; -∆-, pH 7.4) and temperature maintained at 37 °C. (b) In vitro release kinetics of EGCG from uncrosslinked hydrogel in PBS of different pH values (-□-, pH 6.8; -∆-, pH 7.4) and temperature maintained at 37 °C.
Figure 9.
(a) In vitro release kinetics of EGCG from the optimized formulation of crosslinked hydrogel in PBS of different pH values (-○-, pH 1.2; -□-, pH 6.8; -∆-, pH 7.4) and temperature maintained at 37 °C. (b) In vitro release kinetics of EGCG from uncrosslinked hydrogel in PBS of different pH values (-□-, pH 6.8; -∆-, pH 7.4) and temperature maintained at 37 °C.
Based on the
n values presented in
Table 2, the drug release mechanism of cylindrical crosslinked hydrogel at any pH is characterized by anomalous transport, intermediate between Fickian and Case II. However, the
n value at pH 6.8 is closer to 0.45, signifying that anomalous transport at this pH is more of a Fickian diffusion. The drug release mechanism under Fickian diffusion means that the first 60% normalized drug released at any time is described by a constant multiplied by the square root of time. Conversely, in Case II transport, the normalized water uptake at any time is linearly related to time [
40].
Table 2.
Diffusional exponent (n) and constant (k) of cylindrical crosslinked hydrogel at different pH values from a fitted Peppas equation.
Table 2.
Diffusional exponent (n) and constant (k) of cylindrical crosslinked hydrogel at different pH values from a fitted Peppas equation.
| pH | r2 | n | k |
|---|
| 1.2 | 0.9856 | 0.5962 | 3.86 × 10−3 |
| 6.8 | 0.9933 | 0.467 | 6.88 × 10−3 |
| 7.4 | 0.9865 | 0.5284 | 4.92 × 10−3 |
3.5. Instability of Free-EGCG in Solutions
To determine the stability of EGCG in
in vitro simulated GIT conditions, the concentration of free-EGCG in the solution was monitored against time, as shown in
Figure 10. Since the EGCG solution becomes diluted throughout time by replacing the aliquot withdrawn with fresh solution, there is a plot of the expected concentration of EGCG, as its initial concentration was predetermined. This expected concentration of EGCG is compared to the actual concentration of EGCG based on UV-Vis determination. A deviation of the two plots can be interpreted as the corresponding stability of EGCG in the solution at a specific pH. As shown in
Figure 10, EGCG is stable at pH 1.2, proven by the overlapping of its actual and expected concentration curves. However, as the pH becomes higher, the two curves deviate more, with a big difference at pH 7.4. It can be interpreted that EGCG is more unstable in an alkaline solution.
In comparison, the controlled release of EGCG from hydrogel provides stability for EGCG, as shown in
Figure 9a. There is no instance at any pH of the solution where the concentration of EGCG from the releasing medium decreased, even though monitored up to 12 h. This means that the released EGCG is not degraded instantly and, then, is compensated for by the continuous release of EGCG. The only reason why at different pH values the sustained-release curves differ is because of the pH-responsiveness of the hydrogel. It is major concern that free EGCG (or released EGCG) is subject to oxidation in the medium (or physiological fluid in the human body). Hu
et al. reported that EGCG was protected from oxidation by encapsulating polymers of a casein peptides/chitosan mixture. In their report, the enhanced permeation of released EGCG was observed in the
in vitro permeation test of Caco-2 cell monolayers [
41]. This indicates the good stability of EGCG in intestinal fluid. Shutava
et al. utilized a polyelectrolyte layer-by-layer technique to coat EGCG over gelatin core nanoparticles. It was shown that the biological activity of EGCG, blocking the signaling pathway of the breast cancer cell line in their study, remained [
42]. Therefore, the utilization of biopolymers to protect EGCG from oxidation is an essential practice for forming a drug delivery system.
Figure 10.
Concentration of free-EGCG against time at pH 1.2, 6.8 and 7.4 at a temperature maintained at 37 °C.
Figure 10.
Concentration of free-EGCG against time at pH 1.2, 6.8 and 7.4 at a temperature maintained at 37 °C.