Effects of Additives on the Morphology and Performance of PPTA/PVDF in Situ Blend UF Membrane
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. The Synthesis of PVDF/PPTA Blend Casting Solution
2.3. The Preparation of PVDF/PPTA Blend Membrane
2.4. Ternary Phase Diagram of PPTA-PVDF/NMP/Water System

2.5. Characterization of Membrane Morphology and Parameter
2.5.1. Field Emission Scanning Electron Microscopy (FESEM)
2.5.2. Porosity and the Mean Pore Size


2.6. Characterization of Membrane Performance
2.6.1. Permeation Flux (F) and PEG Rejection (R)



2.6.2. Water Contact Angle Measurement (WCA)
3. Results and Discussion
3.1. Thermodynamics of Membrane Formation
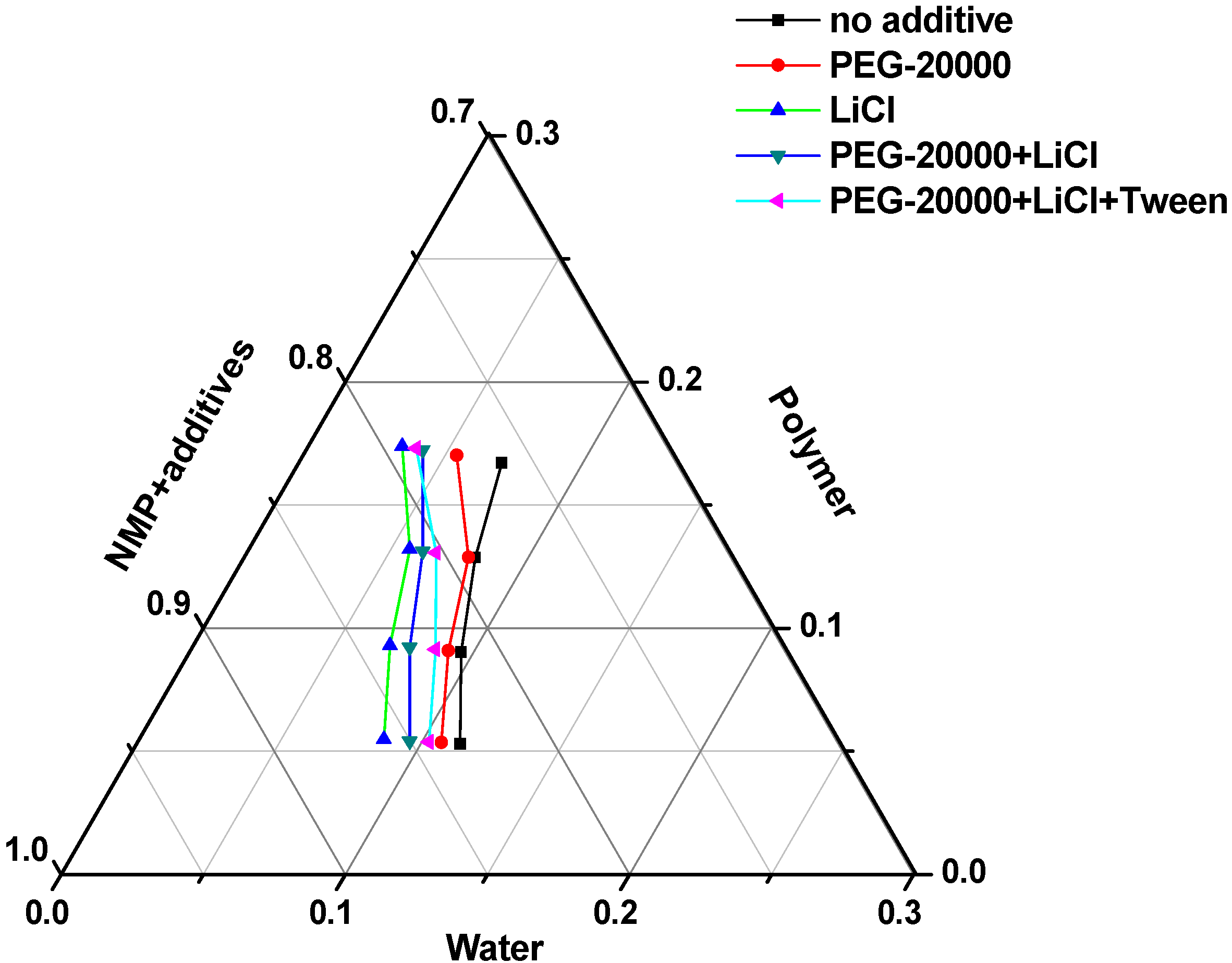

3.2. Kinetics of Membrane Formation
| Additive Content (wt%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Dynamic viscosity (Pa·s) of the dope with LiCl | 15.1 | – | 29.6 | 54.3 | 91.6 | 218.4 | – | – | – |
| Dynamic viscosity (Pa·s) of the dope with PEG | 15.1 | – | 20.1 | – | 43.2 | – | 65.6 | – | 121.0 |
| Dynamic viscosity (Pa·s) of the dope with Tween | 45.1 | 55.6 | 60.2 | 76.5 | 89.1 | – | – | – | – |
3.3. Effects of Additives on Membrane Morphology
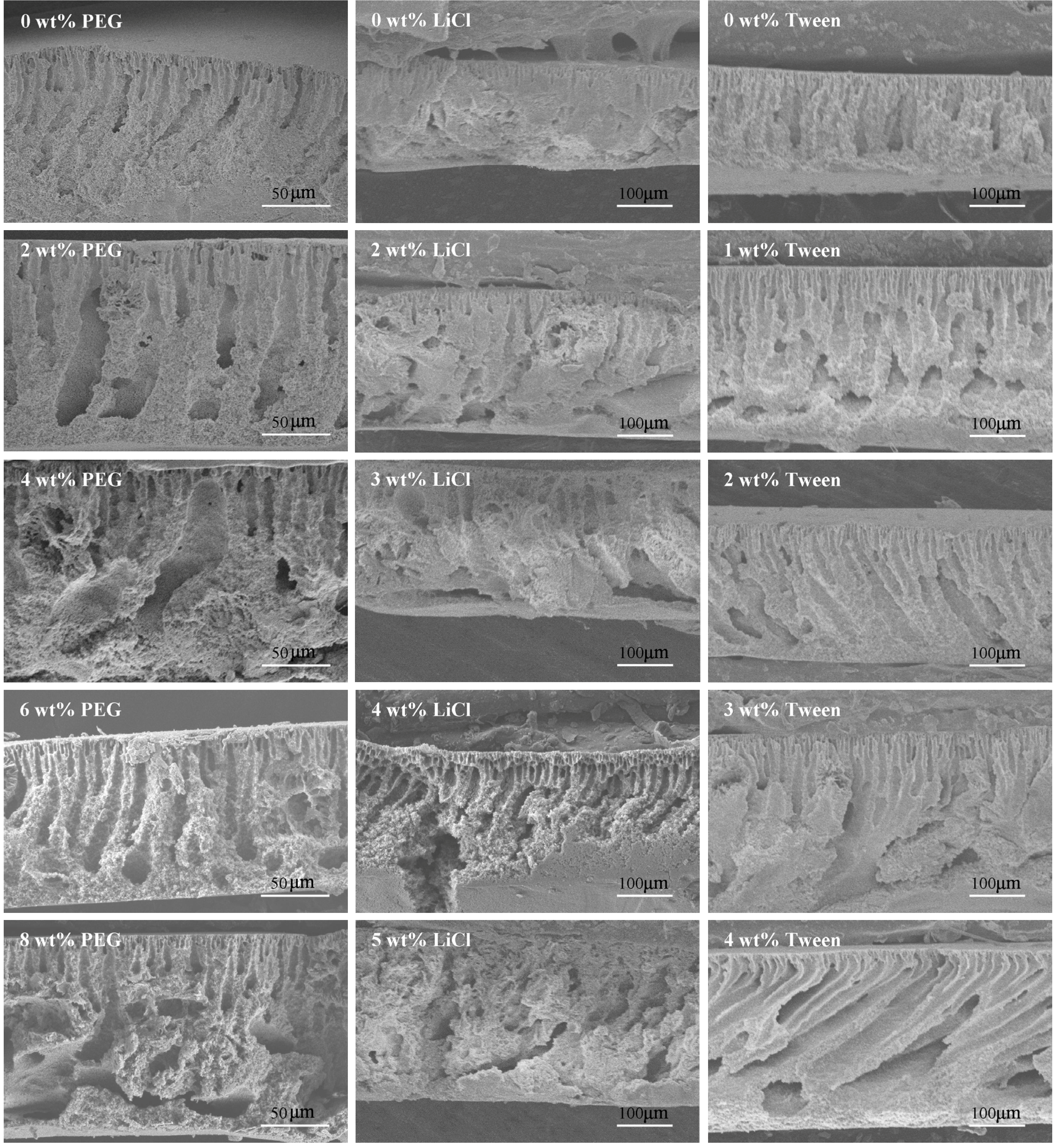
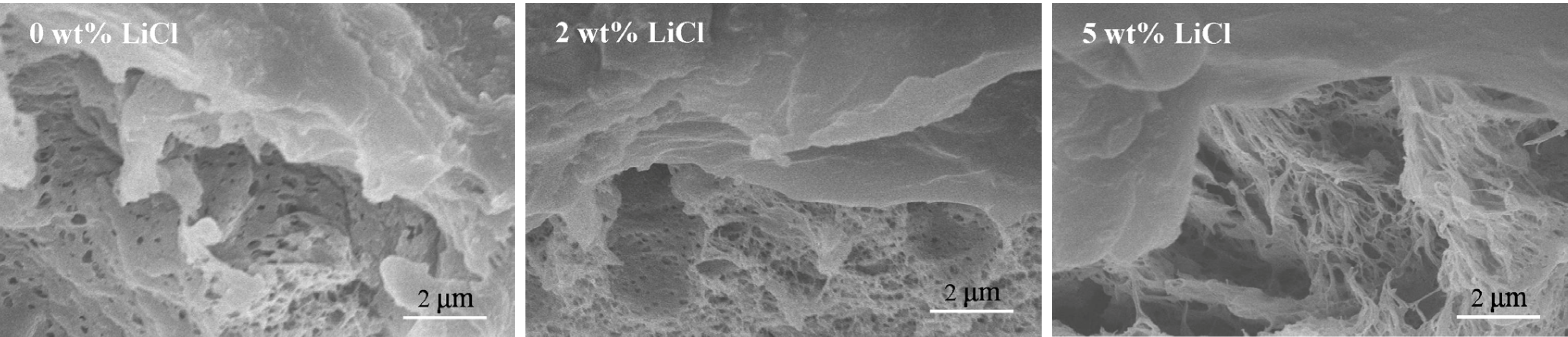

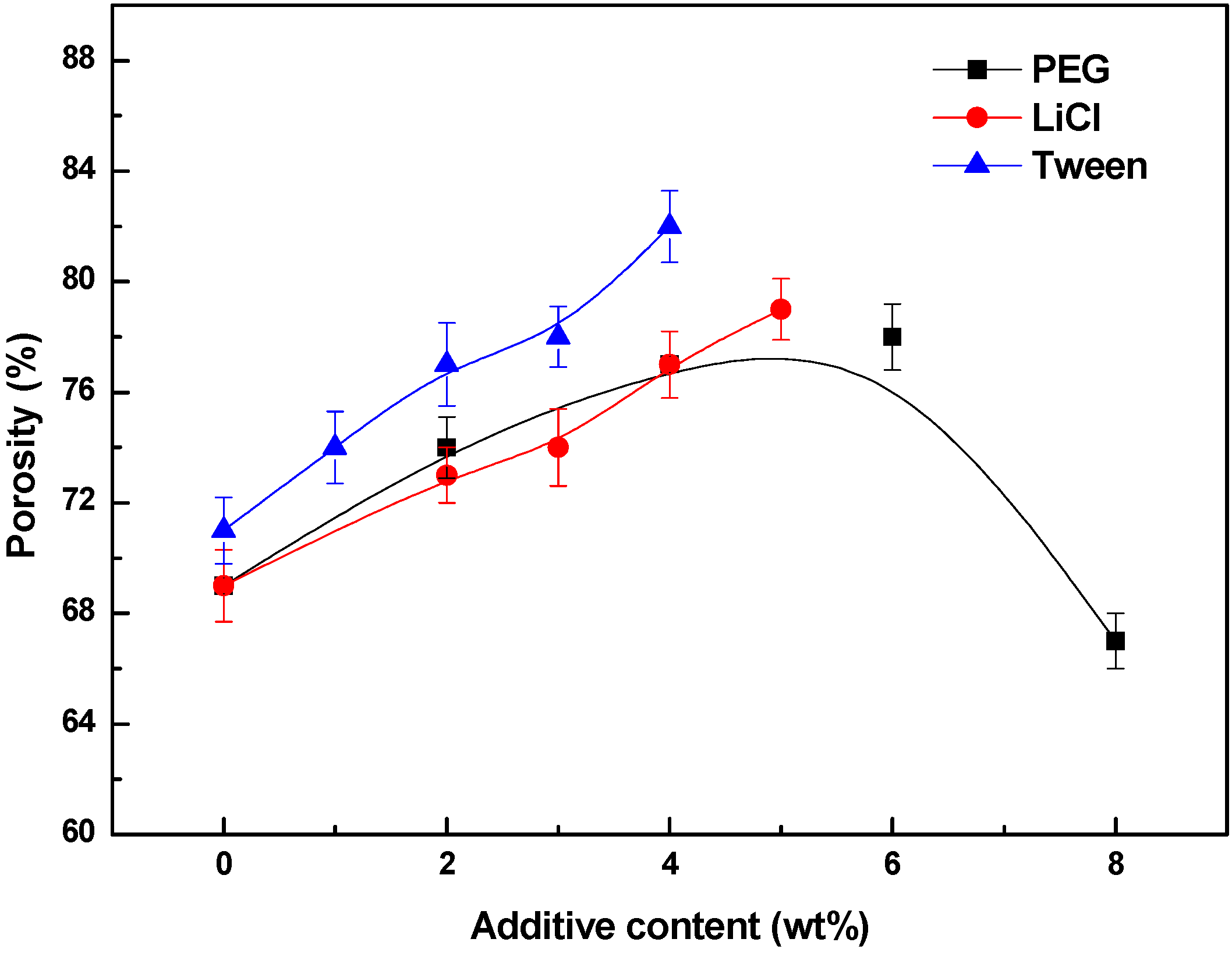
3.4. Effects of Additives on Membrane Performance
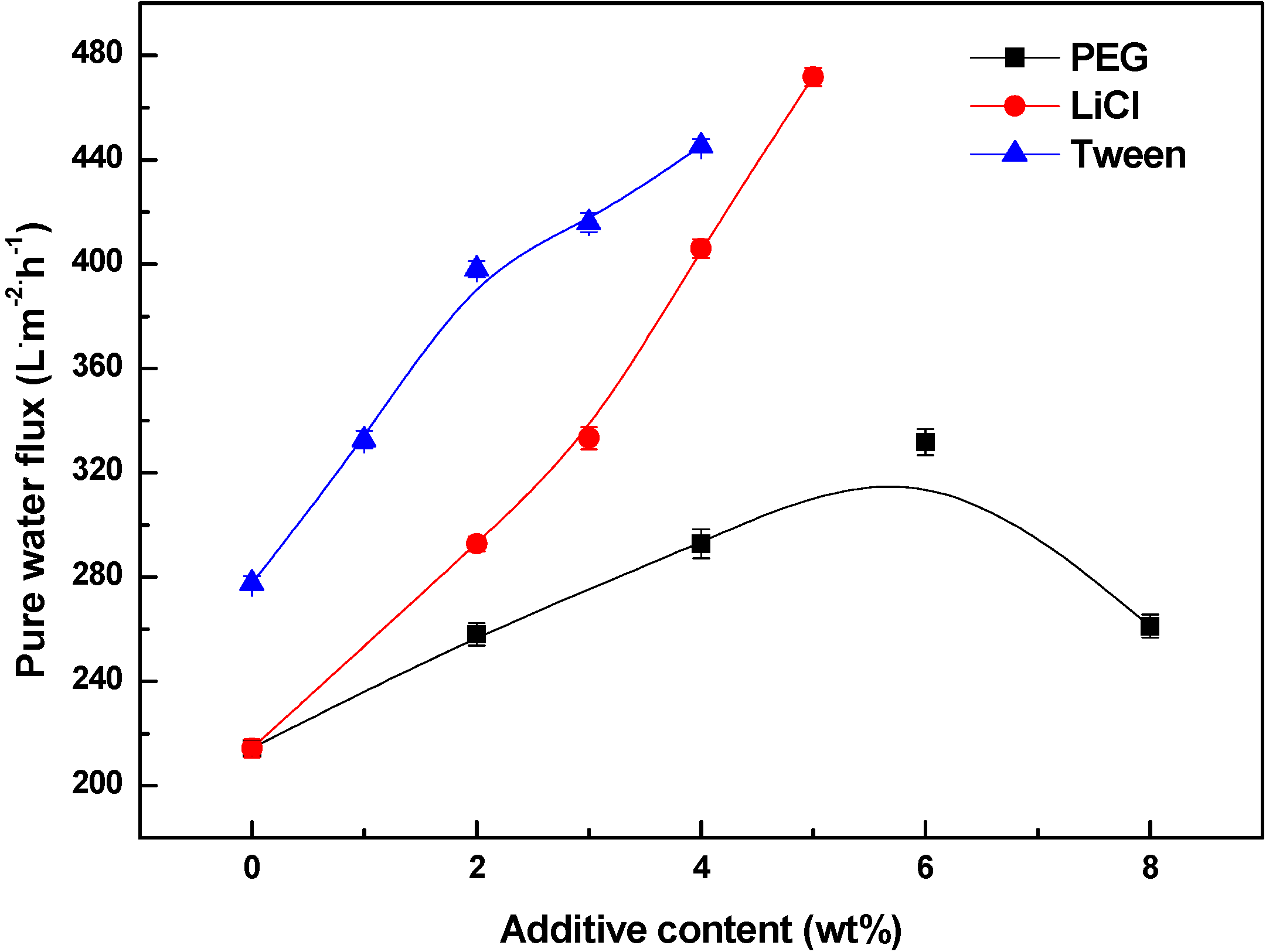
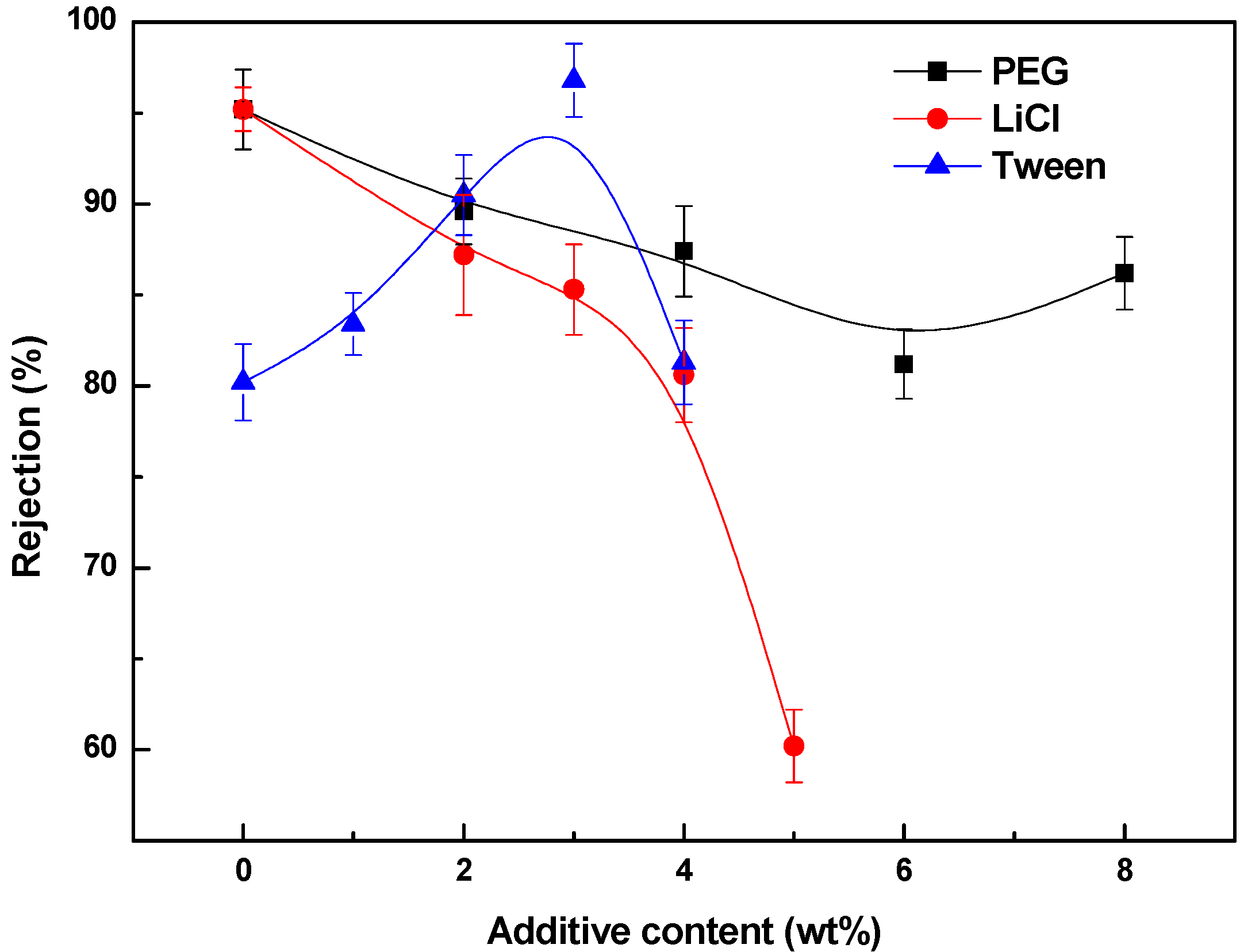
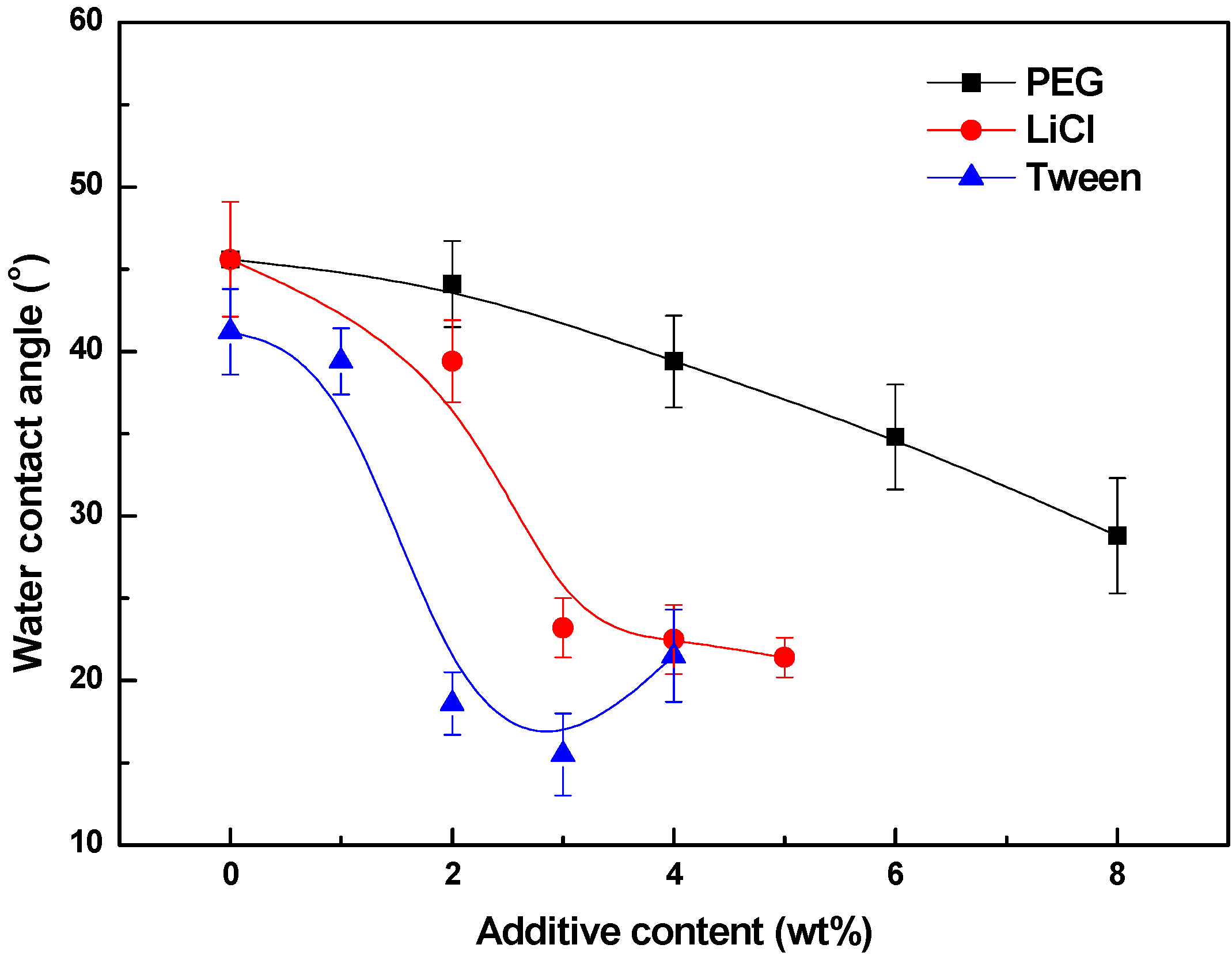
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, F.; Hashim, N.A.; Liu, Y.T.; Moghareh Abed, M.R.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A Review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Dong, J.J.; Liu, D.Q.; Zhang, Y.F.; Wang, C. Preparation and properties of PVDF/PPTA blend membranes. Desalin. Water Treat. 2013, 51, 3814–3820. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Hoek, E.M.V.; Mamba, B.B. Probing the mechanical and thermal properties of polysulfone membranes modified with synthetic and natural polymer additives. Polym. Test. 2014, 34, 202–210. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R. Insight into the role of amphiphilic pluronic block copolymer as pore-forming additive in PVDF membrane formation. J. Membr. Sci. 2013, 446, 492–503. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Membr. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Liu, C.; Yun, Y.B.; Wu, N.J. Effects of amphiphilic additive Pluronic F127 on performance of poly (ether sulfone) ultrafiltration membrane. Desalin. Water Treat. 2013, 51, 3776–3785. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wei, X.; Tian, X.X.; Wang, J.X.; Yang, S.B.; Wang, S.C. Comparison study of the effect of PVP and PANI nanofibers additives on membrane formation mechanism, structure and performance. J. Membr. Sci. 2011, 385–386, 110–122. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.F.; Matsuura, T. Enhanced oil–water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar]
- Zhang, Y.J.; Lin, R.; Yuan, M.Y. Effects of pore-forming additives on structures and properties of PVDF/Fe3+/Cu2+ hollow fiber membranes. Desalin. Water Treat. 2013, 51, 3903–3908. [Google Scholar] [CrossRef]
- Ma, Y.X.; Shi, F.M.; Zhao, W.J.; Wu, M.N.; Zhang, J.; Ma, J.; Gao, C.J. Preparation and characterization of PSf/clay nanocomposite membranes with LiCl as a pore forming additive. Desalination 2012, 303, 39–47. [Google Scholar] [CrossRef]
- Ebert, K.; Fritsch, D.; Koll, J.; Tjahjawiguna, C. Influence of inorganic fillers on the compaction behaviour of porous polymer based membranes. J. Membr. Sci. 2004, 233, 71–78. [Google Scholar] [CrossRef]
- Amirilargani, M.; Saljoughi, E.; Mohammadi, T. Effects of Tween 80 concentration as a surfactant additive on morphology and permeability of flat sheet polyethersulfone (PES) membranes. Desalination 2009, 249, 837–842. [Google Scholar] [CrossRef]
- Yang, Y.N.; Wu, J.; Zheng, Q.Z.; Chen, X.S.; Zhang, H.X. The research of rheology and thermodynamics of organic-inorganic hybrid membrane during the membrane formation. J. Membr. Sci. 2008, 311, 200–207. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, L.; Sun, D.H.; An, Q.F.; Chen, H.L. Formation mechanism and crystallization of poly(vinylidene fluoride) membrane via immersion precipitation method. Desalination 2009, 236, 170–178. [Google Scholar] [CrossRef]
- Barth, C.; Gonçalves, M.C.; Pires, A.T.N.; Roeder, J.; Wolf, B.A. Asymmetric polysulfone and polyethersulfone membranes: effects of thermodynamic conditions during formation on their performance. J. Membr. Sci. 2000, 169, 287–299. [Google Scholar] [CrossRef]
- Huang, Q.L.; Xiao, C.F.; Hu, X.Y.; Li, X.F. Study on the effects and properties of hydrophobic poly(tetrafluoroethylene) membrane. Desalination 2011, 277, 187–192. [Google Scholar] [CrossRef]
- Shi, H.Y.; Liu, F.; Xue, L.X. Fabrication and characterization of antibacterial PVDF hollow fibre membrane by doping Ag-loaded zeolites. J. Membr. Sci. 2013, 437, 205–215. [Google Scholar] [CrossRef]
- Feng, C.S.; Wang, R.; Shi, B.L.; Li, G.M.; Wu, Y.L. Factors affecting pore structure and performance of poly(vinylidene fluoride-co-hexafluoro propylene) asymmetric porous membrane. J. Membr. Sci. 2006, 277, 55–64. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, J.F.; Dai, Z.; Zhao, K.Y.; Zhang, H. Preparation and characterization of negatively charged hollow fiber nanofiltration membrane by plasma-induced graft polymerization. Desalination 2012, 286, 138–144. [Google Scholar] [CrossRef]
- Cheng, L.P. Effect of temperature on the formation of microporous PVDF membranes by precipitation from 1-octanol/DMF/PVDF and water/DMF/PVDF systems. Macromolecules 1999, 32, 6668–6674. [Google Scholar] [CrossRef]
- Lang, W.Z.; Ji, Q.; Shen, J.P.; Guo, Y.J.; Xu, Z.L. The roles of alkali metal counter-ions of PFSA play in the formation of PVDF/PFSA-M hollow fiber membranes. Desalination 2012, 292, 45–52. [Google Scholar] [CrossRef]
- Han, R.L.; Zhang, S.H.; Jian, X.G. Effect of additives on the performance and morphology of copoly (phthalazinone ether sulfone) UF membrane. Desalination 2012, 290, 67–73. [Google Scholar] [CrossRef]
- Tager, A.A.; Dreval, Y.Y.; Serikov, Y.A.; Surayeva, T.V. Effect of inorganic salt additives on the viscosity of cellulose acetate solutions. Polym. Sci. U.S.S.R. 1976, 18, 2163–2168. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Sriyamuna Devi, T.K.; Mohan, D. Development, characterization and separation performance of organic-inorganic membranes: Part II. Effect of additives. Sep. Purif. Technol. 2009, 67, 271–281. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.-B.; Shi, W.-Y.; Zhang, Y.-F.; Liu, D.-Q.; Liu, X.-F. Effects of Additives on the Morphology and Performance of PPTA/PVDF in Situ Blend UF Membrane. Polymers 2014, 6, 1846-1861. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6061846
Li H-B, Shi W-Y, Zhang Y-F, Liu D-Q, Liu X-F. Effects of Additives on the Morphology and Performance of PPTA/PVDF in Situ Blend UF Membrane. Polymers. 2014; 6(6):1846-1861. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6061846
Chicago/Turabian StyleLi, Hong-Bin, Wen-Ying Shi, Yu-Feng Zhang, Dong-Qing Liu, and Xiao-Feng Liu. 2014. "Effects of Additives on the Morphology and Performance of PPTA/PVDF in Situ Blend UF Membrane" Polymers 6, no. 6: 1846-1861. https://0-doi-org.brum.beds.ac.uk/10.3390/polym6061846





