Improved Method for Preparation of Amidoxime Modified Poly(acrylonitrile-co-acrylic acid): Characterizations and Adsorption Case Study
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials and Methods
2.2. Instrumentation
2.3. Polymer Synthesis
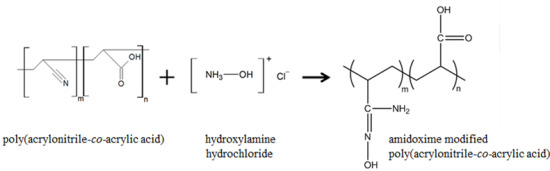
2.4. Modification with Hydroxylamine Hydrochloride
| Method | Refined Method | Method Differences | Advantages | |
|---|---|---|---|---|
| Previous | Refined | |||
| 1 | 0.375 g of hydroxylamine hydrochloride and 0.375 g of sodium hydroxide were diluted with deionized water in a 50 mL beaker together with 0.025 g of poly(AN-co-AA). The mixture was kept for 2 days at room temperature. Then, the poly(AN-co-AA) was filtered, washed, and dried at 60 °C. | The mixture was continuoslystirred for 2 days [12]. | The mixture was soaked for 2 days. | Less energy consumption. |
| 2 | 0.5 M of hydroxylamine hydrochloride (HH) was adjusted to pH 6 by sodium bicarbonate. The mixture was heated and stirred at 70 °C and 80 rpm respectively. 0.250 g of poly(AN-co-AA) was added and allowed to react for 1 h. Then, the poly(AN-co-AA) was filtered, washed, and dried at 60 °C. | The HH needed was 1.2 M for 0.20 g polymer [15]. | The HH needed was 0.5 M for 0.25 g polymer. | Less usage of chemical. |
| 3 | 0.10 g of sodium hydroxide and 0.17 g of hydroxylamine hydrochloride were mixed with 50 mL of deionized water and 0.10 g poly(AN-co-AA). The mixture was kept at 50 °C for 2 h. Then, the poly(AN-co-AA) was filtered, washed, and dried at 60 °C. | The reaction time was 2 days [12]. | The reaction time was 2 h. | Less time reaction. |
| 4 | 1.0 g of poly(AN-co-AA), 25 mL of methanol and 3.0 g of hydroxylamine hydrochloride were mixed and stirred for 2 h at room temperature. Then, 1.8 g of sodium hydroxide was diluted in 6 mL of deionized water and added into the mixture to neutralize hydrochloric acid and kept at pH 8. The mixture was refluxed for 4 h at 70 °C. Then, the poly(AN-co-AA) was filtered, washed, and dried at 60 °C. | The reaction was refluxed for 20 h [4]. | The reaction was refluxed for 4 h. | Less usage of energy and short reaction time. |
2.5. Amine Capacity
3. Results and Discussion
3.1. Synthesis of Polymer

3.2. Actual Composition of Comonomer

| Comonomer feed ratio | Actual composition in poly(AN-co-AA) | ||
|---|---|---|---|
| AN | AA | AN | AA |
| 100 | 0 | 100 | – |
| 97 | 3 | 95 | 5 |
| 95 | 5 | 89 | 11 |
| 93 | 7 | 85 | 15 |
| 90 | 10 | 79 | 21 |
3.3. Amine Capacity

3.4. Fourier Transform Infrared (FTIR) Spectra


3.5. Elemental Microanalysis
| Ratio of AN:AA | 100:0 | 97:3 | 95:5 | 93:7 | 90:10 | |
|---|---|---|---|---|---|---|
| Carbon | a UM | 63.6 | 61.2 | 59.9 | 63.3 | 59.7 |
| b M | 38.8 | 39.0 | 40.9 | 46.0 | 40.6 | |
| Hydrogen | a UM | 6.8 | 6.4 | 6.3 | 6.9 | 6.4 |
| b M | 7.4 | 7.4 | 8.1 | 8.8 | 7.2 | |
| Nitrogen | a UM | 24.5 | 24.1 | 20.9 | 21.1 | 19.4 |
| b M | 24.8 | 24.3 | 24.3 | 26.4 | 22.4 | |
3.6. Scanning Electron Microscopy (SEM)

3.7. Thermogravimetric Analysis (TGA)

3.8. Amidoxime (AO) Modified Poly(AN-co-AA)
3.9. Case Study on Adsorption Application
| Ratio AN:AA of AO Modified Poly(AN-co-AA) | Cadmium Ions (Cd2+) | Lead Ions (Pb2+) | ||
|---|---|---|---|---|
| Percentage Removal, Qe (%) | Standard Deviation | Percentage Removal, Qe (%) | Standard Deviation | |
| 100:0 | 21 | ±0.6 | 80 | ±0.8 |
| 97:3 | 15 | ±0.3 | 70 | ±1.1 |
| 95:5 | 21 | ±0.7 | 81 | ±0.7 |
| 93:7 | 38 | ±0.5 | 87 | ±0.2 |
| 90:10 | 24 | ±1.0 | 74 | ±0.5 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mishra, A.; Sharma, S.; Gupta, B. Studies on the amidoximation of polyacrylonitrile films: Influence of synthesis conditions. J. Appl. Polym. Sci. 2011, 121, 2705–2709. [Google Scholar] [CrossRef]
- Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Redox copolymerization of acrylonitrile with fumaronitrile as a precursor for carbon fibre. J. Polym. Res. 2007, 14, 379–385. [Google Scholar] [CrossRef]
- Kiani, G.R.; Sheikhloie, H.; Arsalani, N. Heavy metal ion removal from aqueous solutions by functionalized polyacrylonitrile. Desalination 2011, 269, 266–270. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Wang, C.; Qu, R.; Ji, C.; Sun, C.; Zhang, Y. Synthesis of porous acrylonitrile/methyl acrylate copolymer beads by suspended emulsion polymerization and their adsorption properties after amidoximation. J. Hazard. Mater. 2010, 175, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Cheng, G.; Zhu, D.; Xue, H.; Cosnier, S.; Ding, S. Direct electrochemistry of hemoglobin in poly(acrylonitrile-co-acrylic acid) and its catalysis to H2O2. Sens. Actuators B 2009, 137, 259–265. [Google Scholar] [CrossRef]
- Bhanu, V.A.; Rangarajan, P.; Wiles, K.; Bortner, M.; Sankarpandian, M.; Godshall, D.; Glass, T.E.; Banthia, A.K.; Yang, J.; Wilkes, G.; et al. Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors. Polymer 2002, 43, 4841–4850. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ngho, Y.C. Adsorption of UO22+ by polyethylene adsorbents with amidoxime, carboxyl, and amidoxime/carboxyl group. Radiat. Phys. Chem. 2000, 57, 187–193. [Google Scholar] [CrossRef]
- Shaaban, A.F.; Fadel, D.A.; Mahmoud, A.A.; Elkomy, M.A.; Elbahy, S.M. Synthesis of a new chelating resin bearing amidoxime group for adsorption of Cu(II), Ni(II) and Pb(II) by batch and fixed-bed column methods. J. Environ. Chem. Eng. 2014, 2, 632–641. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Igberase, E.; Osifo, P. Equilibrium, kinetic, thermodynamic and desorption studies of cadmium and lead by polyaniline grafted cross-linked chitosan beads from aqueous solution. J. Ind. Eng. Chem. 2014, 26, 6–13. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Horzum, N.; Shahwan, T.; Parlak, O.; Demir, M.M. Synthesis of amidoximated polyacrylonitrile fibers and its application for sorption of aqueous uranyl ions under continuous flow. Chem. Eng. J. 2012, 213, 41–49. [Google Scholar] [CrossRef]
- Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Preparation and thermal behaviour of acrylonitrile (AN)/Ethyl acrylate (EA) copolymer and acrylonitrile (AN)/ethyl acrylate (EA)/fumaronitrile (FN) Terpolymer as precursors for carbon fibre. Pertanika J. Sci. Technol. 2010, 18, 401–409. [Google Scholar]
- Jamil, S.N.A.M.; Daik, R.; Ahmad, I. Redox synthesis and thermal behavior of acyrlonitrile-methyl acrylate-fumaronitrile terpolymer as precursor for carbon fibre. Int. J. Chem. Eng. Appl. 2012, 3, 416–420. [Google Scholar]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A. K. Acrylonitrile-acrylic acids copolymers. I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Akbari, S.; Kish, M.H.; Entezami, A.A. Copolymer of acrylonitrile/acrylic acid film dendrigrafted with citric acid: host/guest properties of dendrigraft/dye complexes in relation to acrylic acid content. Iran. Polym. J. 2011, 20, 539–549. [Google Scholar]
- Moghadam, S.S.; Bahrami, S.H. Copolymerization of acrylonitrile-acrylic acid in DMF-water mixture. Iran. Polym. J. 2005, 14, 1032–1041. [Google Scholar]
- Sahoo, A.; Ramasubramani, K.R.T.; Jassal, M.; Agrawal, A.K. Effect of copolymer architecture on the response of pH sensitive fibers based on acrylonitrile and acrylic acid. Eur. Polym. J. 2007, 43, 1065–1076. [Google Scholar] [CrossRef]
- Mishra, M.K.; Yagci, Y. Handbook of Vinyl Polymers: Radical Polymerization, Process, and Technology, 2nd ed.; CRC Press: Boca Raton, Florida, FL, USA, 2008. [Google Scholar]
- Ebewele, R.O. Polymer Science and Technology; CRC Press: Boca Raton, Florida, FL, USA, 2000. [Google Scholar]
- Dong, Y.; Han, Z.; Liu, C.; Du, F. Preparation and photocatalytic performance of Fe (III)-amidoximated PAN fiber complex for oxidative degradation of azo dye under visible light irradiation. Sci. Total Environ. 2010, 408, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- El-shishtawy, R.M.; Ahmed, N.S.E. Anionic coloration of acrylic fibre. Part 1: Efficient pretreatment and dyeing with acid dyes. Color. Techol. 2005, 121, 139–146. [Google Scholar] [CrossRef]
- Choi, S.-H.; Choi, M.-S.; Park, Y.-T.; Lee, K.-P.; Kang, H.-D. Adsorption of uranium ions by resins with amidoxime and amidoxime/carboxyl group prepared by radiation-induced polymerization. Radiat. Phys. Chem. 2003, 67, 387–390. [Google Scholar] [CrossRef]
- Venugopal, A.P.; Cespedes, O.; Russell, S.J. Controlling dielectric and magnetic properties of PVdF/magnetite nanocomposite fibre webs. Int. J. Polym. Sci. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Cormack, P.A.G.; Sherrington, D.C.; Khoshdel, E. Synthesis and characterization of micrometer-sized molecularly imprinted spherical polymer particulates prepared via precipitation polymerization. Pure Appl. Chem. 2007, 79, 1505–1519. [Google Scholar] [CrossRef]
- Coleman, M.M.; Sivy, G.T. Fourier transform IR studies of the degradation of polyacrylonitrile copolymers-I. Introduction and comparative rates of the degradation of three copolymers below 200 °C and under reduced pressure. Carbon 1981, 19, 123–126. [Google Scholar] [CrossRef]
- McCaugh, M.C.; Kottle, S. The thermal degradation poly(acrylic acid). J. Polym. Sci. Part B 2003, 5, 817–820. [Google Scholar] [CrossRef]
- Chen, R.; Chen, D.; Su, S. Synthesis and characterization of novel oligomeric poly(styrene-co-acrylonitrile)-clay complexes. J. Appl. Polym. Sci. 2009, 112, 3355–3361. [Google Scholar] [CrossRef]
- Grassie, N.; McGuchan, R. Pyrolysis of polyacrylonitrile and related polymers-VI. Acrylonitrile copolymers containing carboxylic acid and amide structures. Eur. Polym. J. 1972, 8, 257–269. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdel-Rahman, A.A.-H.; El-Aasy, I.E.; Ahmed, F.Y.; Hamza, M.F. Adsorption properties of uranium(VI) ions on reaction crossliked acrylamidoxime and acrylic acid copolymer resin. J. Dispers. Sci. Technol. 2010, 32, 84–94. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Zahri, N.A.; Md Jamil, S.N.A.; Abdullah, L.C.; Shean Yaw, T.C.; Nourouzi Mobarekeh, M.; Sim, J.H.; Mohd Rapeia, N.S. Improved Method for Preparation of Amidoxime Modified Poly(acrylonitrile-co-acrylic acid): Characterizations and Adsorption Case Study. Polymers 2015, 7, 1205-1220. https://0-doi-org.brum.beds.ac.uk/10.3390/polym7071205
Mohd Zahri NA, Md Jamil SNA, Abdullah LC, Shean Yaw TC, Nourouzi Mobarekeh M, Sim JH, Mohd Rapeia NS. Improved Method for Preparation of Amidoxime Modified Poly(acrylonitrile-co-acrylic acid): Characterizations and Adsorption Case Study. Polymers. 2015; 7(7):1205-1220. https://0-doi-org.brum.beds.ac.uk/10.3390/polym7071205
Chicago/Turabian StyleMohd Zahri, Nur Amirah, Siti Nurul Ain Md Jamil, Luqman Chuah Abdullah, Thomas Choong Shean Yaw, Mohsen Nourouzi Mobarekeh, Jia Huey Sim, and Nur Salimah Mohd Rapeia. 2015. "Improved Method for Preparation of Amidoxime Modified Poly(acrylonitrile-co-acrylic acid): Characterizations and Adsorption Case Study" Polymers 7, no. 7: 1205-1220. https://0-doi-org.brum.beds.ac.uk/10.3390/polym7071205