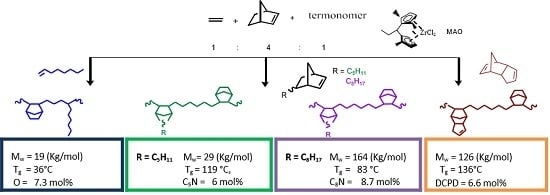
Terpolymerization of Substituted Cycloolefin with Ethylene and Norbornene by Transition Metal Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations and Materials
2.2. Analytical Measurements
2.3. 5-Alkyl-2-Norbornene Synthesis
2.4. Polymerization Procedure
2.5. Monomers Content Determination
3. Results and Discussion
3.1. Activities
3.2. Microstructure
3.3. Molar Masses
3.4. Chain End Analysis
3.5. Thermal Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Busico, V. Ziegler-Natta catalysis: Forever young. MRS Bull. 2013, 38, 224–228. [Google Scholar]
- Gahleitner, M.; Resconi, L.; Doshev, P. Heterogeneous Ziegler-Natta, metallocene, and post-metallocene catalysis: Successes and challenges in industrial application. MRS Bull. 2013, 38, 229–233. [Google Scholar] [CrossRef]
- Mulhaupt, R. Catalytic polymerization and post polymerization catalysis fifty years after the discovery of Ziegler’s catalyst. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar] [CrossRef]
- Kaminsky, W. Polymerization catalysis. Catal. Today 2000, 62, 23–24. [Google Scholar] [CrossRef]
- Kaminsky, W.; Laban, A. Metallocene catalysis. Appl. Catal. 2001, 222, 47–61. [Google Scholar] [CrossRef]
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Chem. A 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt-Rosenau, M. Homo- and copolymerization of cycloolefins by metallocene catalysts; Scheirs, J., Kaminsky, W., Eds.; Wiley: Chichester, West Sussex, 2000. [Google Scholar]
- Kaminsky, W.; Bark, A.; Arndt, M. New polymers by homogenous zirconocene/aluminoxane catalysts. Makromol. Chem. Macromol. Symp. 1991, 47, 83–93. [Google Scholar] [CrossRef]
- Kaminsky, W.; Boggioni, L.; Tritto, I. Cycloolefin polymerization. In Chain Vinyl Polymerization; Coates, G.W., Sawamoto, M., Eds.; Elsevier: Philadelphia, PA, USA, 2012; pp. 843–873. [Google Scholar]
- Boggioni, L.; Tritto, I. State of the art of cyclic olefin polymers. MRS Bull. 2013, 38, 245–251. [Google Scholar] [CrossRef]
- Boggioni, L.; Tritto, I. Polyolefins with cyclic comonomers. Adv. Polym. Sci. 2013, 258, 117–142. [Google Scholar]
- Blank, F.; Janiak, C. Metal catalysts for the vinyl/addition polymerization of norbornene. Coord. Chem. Rev. 2009, 253, 827–861. [Google Scholar] [CrossRef]
- Li, X.; Hou, Z.M. Organometallic catalysts for copolymerization of cyclic olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Ferro, D.R. Metallocene catalyzed ethene- and propene co-norbornene polymerization: Mechanisms from a detailed microstructural analysis. Coord. Chem. Rev. 2006, 250, 212–241. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Ethene-norbornene copolymer with high norbornene content produced by ansa-fluorenylamidodimethyltitanium complex using a suitable activator. Macromolecules 2004, 37, 8503–8509. [Google Scholar] [CrossRef]
- Chen, M.; Zou, W.; Cai, Z.; Chen, C. Norbornene homopolymerization and copolymerization with ethylene by phosphine-sulfonate nickel catalysts. Polym. Chem. 2015, 6, 2669–2676. [Google Scholar] [CrossRef]
- Terao, H.; Iwashita, A.; Ishii, S.; Tanaka, H.; Yoshida, Y.; Mitani, M.; Fujita, T. Ethylene/norbornene copolymerization nehavior of bis(phenoxy-imine)Ti complexes combined with MAO. Macromolecules 2009, 42, 4359–4361. [Google Scholar] [CrossRef]
- Nomura, K.; Yamada, J.; Wang, W.; Liu, J. Effect of ketimide ligand for ethylene polymerization and ethylene/norbornene copolymerization catalyzed by (cyclopentadienyl)(ketimide)titanium complexes–MAO catalyst systems: Structural analysis for Cp∗ TiCl2(N CPh2). J. Organomet. Chem. 2007, 1, 4675–4682. [Google Scholar] [CrossRef]
- Barnes, D.A.; Benedikt, G.M.; Goodall, B.L.; Huang, S.S.; Kalamarides, H.A.; Lenhard, S.; McIntosh, L.H.; Selvy, K.T.; Shick, R.A.; Rhodes, L.F. Addition polymerization of norbornene-type monomers using neutral nickel complexes containing fluorinated aryl ligands. Macromolecules 2003, 36, 2623–2632. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Sacchi, M.C.; Locatelli, P. Cyclic olefin polymerization and relationships between addition and ring opening metathesis polymerization. J. Mol. Catal. A 1998, 133, 139–150. [Google Scholar] [CrossRef]
- Ruchatz, D.; Fink, G. Ethene−Norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 2. Comparative study of different metallocene- and half-sandwich/methylaluminoxane catalysts and analysis of the copolymers by 13C nuclear magnetic resonance spectroscopy. Macromolecules 1998, 31, 4674–4680. [Google Scholar] [PubMed]
- Provasoli, A.; Ferro, D.R.; Tritto, I.; Boggioni, L. The conformational characteristics of ethylene−norbornene copolymers and their influence on the 13C NMR spectra. Macromolecules 1999, 32, 6697–6706. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Zetta, L.; Provasoli, A.; Ferro, D.R. Ethylene−norbornene copolymer microstructure. Assessment and advances based on assignments of 13C NMR spectra. Macromolecules 2000, 33, 8931–8944. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Brintzinger, H.H.; Ferro, D.R. Stereoregular and stereoirregular alternating ethylene−norbornene copolymers. Macromolecules 2001, 34, 5770–5777. [Google Scholar] [CrossRef]
- Thorshaug, K.; Mendichi, R.; Boggioni, L.; Tritto, I.; Trinkle, S.; Friedrich, C.; Mülhaupt, R. Poly(ethene-co-norbornene) obtained with a constrained geometry catalyst. A study of reaction kinetics and copolymer properties. Macromolecules 2002, 35, 2903–2911. [Google Scholar] [CrossRef]
- Herfert, N.; Montag, P.; Fink, G. Elementary processes of the Ziegler catalysis, 7. Ethylene, α-olefin and norbornene copolymerization with the stereorigid catalyst systems Ipr[FluCp]Zrcl2/MAO and Me2Si[Ind]2ZrCl2/MAO. Makromol. Chem. 1993, 194, 3167–3182. [Google Scholar] [CrossRef]
- Rische, T.; Waddon, A.J.; Dickinson, L.C.; MacKnight, W.J. Microstructure and morphology of cycloolefin copolymers. Macromolecules 1998, 31, 1871–1874. [Google Scholar] [CrossRef]
- Tanaka, R.; Kamei, I.; Cai, ZG; Nakayama, Y.; Shiono, T. Ethylene-propylene copolymerization behavior of ansa-dimethylsilylene(fluorenyl)(amido)dimethyltitanium complex: Application to ethylene-propylene-diene or ethylene-propylene-norbornene terpolymers. J. Polym. Chem. A 2015, 53, 685–691. [Google Scholar] [CrossRef]
- Marconi, R.; Boggioni, L.; Ravasio, A.; Di Colo, F.; Tritto, I.; Stehling, U.M. Terpolymerization of linear and alicyclic R-olefins with norbornene and ethylene by ansa-metallocene catalysts. Macromolecules 2011, 44, 795–804. [Google Scholar] [CrossRef]
- Tritto, I.; Ravasio, A.; Boggioni, L.; Bertini, F.; Hitzbleck, J.; Okuda, J. Hydroxyl-functionalized norbornene based co- and terpolymers by scandium half-sandwich catalyst. Macromol. Chem. Phys. 2010, 211, 897–904. [Google Scholar] [CrossRef]
- Liu, S.J.; Yao, Z.; Cao, K.; Li, B.G.; Zhu, S.P. Preparation of polar ethylene-norbornene copolymers by metallocene terpolymerization with triisobutylaluminium-protected but-3-en-1-ol. Macromol. Rap. Commun. 2009, 30, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Chirik, P.J.; Bercaw, J.E. Cyclopentadienyl and olefin substituent effects on insertion and β-hydrogen elimination with group 4 metallocenes. Kinetics, mechanism, and thermodynamics for zirconocene and hafnocene alkyl hydride derivatives. Organometallics 2005, 24, 5407–5423. [Google Scholar] [CrossRef]
- Palmová, I.; Kosek, J.; Schöngut, J.; Marek, M.; Štepánek, K. Experimental and modeling studies of oligomerization and copolymerization of dicyclopentadiene. Chem. Eng. Sci. 2001, 56, 927–935. [Google Scholar] [CrossRef]
- Hong, M.; Pan, L.; Ye, W.P.; Song, D.P.; Li, Y.S. Facile, efficient functionalization of polyethylene via regioselective copolymerization of ethylene with cyclic dienes. J. Polym. Sci. Part A 2010, 48, 1764–1772. [Google Scholar] [CrossRef]
- Cho, E.S.; Joung, U.G.; Lee, B.Y.; Lee, H.; Park, Y-W.; Lee, C.H.; Shin, D.M. Syntheses of 2,5-dimethylcyclopentadienyl ansa-zirconocene complexes and their reactivity for ethylene/norbornene copolymerization. Organometallics 2004, 23, 4693–4699. [Google Scholar] [CrossRef]
- Sagane, T.; Mizuno, A. Synthesis and characterization of poly(5-alkyl-2-norbornene)s by cationic polymerization. Effect of alkyl substituent length on monomer reactivity, polymer structure and thermal properties. Makromol. Chem. 1993, 194, 37–52. [Google Scholar]
- Note: In general the endo isomer, the kinetic product, is more abundant in the mixture. The synthesis of C5N led to the formation of exo/endo isomers = 20/80. The synthesis of C8N, conducted at higher temperature and with longer reaction time, allowed to obtain a higher amount of the thermodynamic exo product and to exo/endo ratio of C8N = 40/60.
- Funk, J.K.; Andes, C.E.; Sen, A. Addition polymerization of functionalized norbornenes: the effect of size, stereochemistry, and coordinating ability of the substituent. Organometallics 2004, 23, 1680–1683. [Google Scholar] [CrossRef]
- Reinmuth, A.; Mathew, J.P.; Melia, J.; Risse, W. (η3-allyl)palladium(II) catalysts for the addition polymerization of norbornene derivatives with functional groups. Macromol. Rapid Commun. 1996, 17, 173–180. [Google Scholar] [CrossRef]
- Mathew, J.P.; Reinmuth, A.; Melia, J.; Swords, N.; Risse, W. (η3-Allyl)palladium(II) and palladium(II) nitrile catalysts for the addition polymerization of norbornene derivatives with functional groups. Macromolecules 1996, 29, 2755–2763. [Google Scholar] [CrossRef]
- Simanke, A.G.; Mauler, S.R.; Galland, G.B. Ethylene copolymerization with cyclic dienes using rac-Et[Ind]2ZrCl2–methylaluminoxane. J. Polym. Sci. Part A 2002, 40, 471–485. [Google Scholar] [CrossRef]
- Note: The endo/exo ratio in C5N and C8N terpolymers was calculated from the peak areas of C1 signals (endo at 30.57 ppm and exo at 31.17 ppm). The endo/exo ratio in C5N terpolymers prepared with catalyst 1 and 2, are 33/67 and 20/80, respectively; the endo/exo ratio in C8N terpolymers prepared with catalyst 1 and 2, are 50/50 and 24/76, respectively. The exo isomers are the most reactive especially with catalyst 2.
- Bhriain, N.N.; Brintzinger, H.H.; Ruchatz, D.; Fink, G. Polymeryl exchange between ansa-zirconocene catalysts for norbornene−ethene copolymerization and aluminum or zinc alkyls. Macromolecules 2005, 38, 2056–2063. [Google Scholar]
- Busico, V.; Cipullo, R.; Friederichs, N.; Linssen, H.; Segre, A.; Castelli, V.V.; van der Velden, G. H1 NMR analysis of chain unsaturations in ethene/1-octene copolymers prepared with metallocene catalysts at high temperature. Macromolecules 2005, 38, 6988–6996. [Google Scholar] [CrossRef]
- Karafilidis, C.; Angermund, K.; Gabor, B.; Rufinska, A.; Mynott, R.J.; Breitenbruch, G.; Thiel, W.; Fink, G. Helical microstructure of polynorbornene. Angew. Chem. Int. Ed. 2007, 46, 3745–3749. [Google Scholar] [CrossRef] [PubMed]












| Region | ppm | Poly(E-ter-N-ter-C5N) | Poly(E-ter-N-ter-C8N) | Poly(E-ter-N-ter-DCPD) |
|---|---|---|---|---|
 |  |  | ||
| A | 12.03 to 12.05 | 1B5 | 1B8 | |
| 20.67 to 20.90 | 2B5 | 2B8 | ||
| B | 25.00 to 36.25 | C5/C6; C6'; CH2 (E); 3B5; 4B5; C7; C7'; 5B5 | C5/C6; C6'; CH2 (E); 3B8; 4B8; 5B8; 6B8; 7B8 | C5/C6; CH2 (E); C7; |
| C7; C7'; 8B8 | ||||
| 33.4 | C5' | |||
| 34.35 | C10' | |||
| C | 36.26 to 50.00 | C1/C4; C1'/C4' | C1/C4; C1'/C4' | C1/C4; C1'/C7'; C2/C3; C8'/C9' |
| C2/C3; C2'/C3'; C5' | C2/C3; C2'/C3'; C5' | |||
| 40.91 | C6' | |||
| 51.72 | C2' | |||
| 128.88 | C4' | |||
| 130.64 | C3' |
| Entry a | Catalyst | Termonomer | N/E/Termonomer b | Activity (kg/mol h atm) | N (mol %) c | Termonomer (mol %) c | Cycloolefin content (%) | Tg d | Mw (kg/mol) e | Mw/Mn e | DP f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | / | 2/1/0 | 2,000 | 31.0 | 0 | 31.0 | 66 | 370 | 1.6 | 4,860 |
| 2 | 1 | / | 4/1/0 | 1,830 | 39.6 | 0 | 39.6 | 100 | 461 | 1.7 | 5,300 |
| 3 | 1 | 1-Octene | 4/1/1 | 1,020 | 38.1 | 3.0 | 38.1 | 25 | 11 | 1.8 | 108 |
| 4 | 1 | 1-Octene | 8/1/1 | 370 | 42.5 | 2.6 | 42.5 | 66 | 11 | 1.7 | 110 |
| 5 | 1 | C5N | 4/1/1 | 100 | 19.8 | 3.4 | 23.2 | 76 | 70 | 2.2 | 434 |
| 6 | 1 | C8N | 4/1/1 | 150 | 22.0 | 7.0 | 29.0 | 64 | 66 | 2.2 | 535 |
| 7 | 1 | DCPD | 4/1/1 | 120 | 30.4 | 3.6 | 34.0 | 101 | 83 | 1.6 | 1,022 |
| 8 | 2 | / | 2/1/0 | 1,920 | 36.6 | 0 | 36.6 | 87 | 157 | 2.1 | 1,433 |
| 9 | 2 | / | 4/1/0 | 1,700 | 45.0 | 0 | 45.0 | 129 | 195 | 2.1 | 1,649 |
| 10 | 2 | / | 8/1/0 | 1,230 | 53.7 | 0 | 53.7 | 164 | 220 | 2.4 | 1,439 |
| 11 | 2 | 1-Octene | 4/1/1 | 1,400 | 45.8 | 7.3 | 45.8 | 36 | 19 | 1.9 | 151 |
| 12 | 2 | 1-Octene | 8/1/1 | 500 | 48.2 | 6.0 | 48.2 | 86 | 35 | 1.7 | 309 |
| 13 | 2 | C5N | 4/1/1 | 380 | 41.5 | 6.7 | 48.2 | 119 | 29 | 1.7 | 268 |
| 14 | 2 | C8N | 4/1/1 | 880 | 37.4 | 8.7 | 46.1 | 83 | 164 | 1.6 | 1,536 |
| 15 | 2 | DCPD | 4/1/1 | 550 | 44.7 | 6.6 | 51.3 | 136 | 126 | 2.5 | 783 |
| 16 | 2 | C5N | 8/1/1 | 290 | 47.5 | 6.1 | 53.6 | 139 | 21 | 1.6 | 199 |
| 17 | 2 | C8N | 8/1/1 | 1,000 | 46.3 | 6.3 | 52.6 | 131 | 125 | 1.6 | 1,103 |
| 18 | 2 | DCPD | 8/1/1 | 180 | 53.8 | 3.5 | 57.3 | 152 | 91 | 2.1 | 640 |
| Structure | Notation | 1H NMR (ppm) | Structure | Notation | 1H NMR (ppm) |
|---|---|---|---|---|---|
 | Vy-1 | a: 4.80 to 4.93d: 5.72 |  | Nx-2 | a: 5.38 to 5.39 |
 | Vy-2 | a: 4.74 to 4.85d: 5.69 |  | Nx-1 | b: 5.56 to 5.57 |
 | Vyx-2 | a:4.74 to 4.85d: 5.63 |  | N-1 | e: 5.44 to 5.50 |
 | O-1 | b: 5.11 [44] |  | N-2 | c: 5.30 |
 | O-2 | b: 5.09 [44] |  | O-4 | f: 4.68 [44] |
 | O-3 | b: 5.08 [44] |  | O-5 | f: 4.73 [44] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggioni, L.; Galotto Galotto, N.; Bertini, F.; Tritto, I. Terpolymerization of Substituted Cycloolefin with Ethylene and Norbornene by Transition Metal Catalyst. Polymers 2016, 8, 60. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030060
Boggioni L, Galotto Galotto N, Bertini F, Tritto I. Terpolymerization of Substituted Cycloolefin with Ethylene and Norbornene by Transition Metal Catalyst. Polymers. 2016; 8(3):60. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030060
Chicago/Turabian StyleBoggioni, Laura, Nella Galotto Galotto, Fabio Bertini, and Incoronata Tritto. 2016. "Terpolymerization of Substituted Cycloolefin with Ethylene and Norbornene by Transition Metal Catalyst" Polymers 8, no. 3: 60. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030060