Novel Functionalized Polythiophene-Coated Fe3O4 Nanoparticles for Magnetic Solid-Phase Extraction of Phthalates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard, Reagents and Chemicals
2.2. Instruments
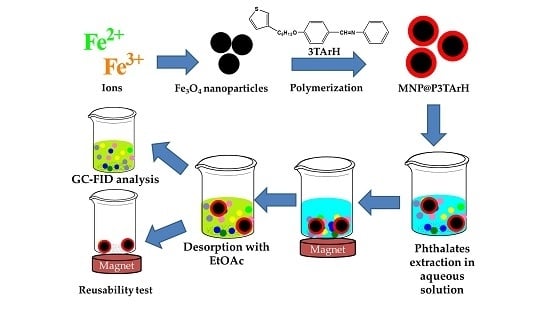
2.3. Synthesis of Adsorbents
2.3.1. Synthesis of (Phenyl-(4-(6-Thiophen-3-yl-Hexyloxy)-Benzylidene)-Amine) Monomer (3) (3TArH)
- •
- 3-(6-bromohexyl)thiophene (1): 3-bromothiophene (2 mL, 21.3 mmol) was added to the dry, degassed hexane (50 mL). The reaction started by cooling the flask at −78 °C. n-Butyllithium in hexane (10.16 mL) was poured into the reaction flask and stirred for 10 min. THF (5 mL) was injected drop-wise for 15 min and continuously stirred for 1 h, which produced a white precipitate and clear supernatant liquid. The supernatant liquid was removed and changed with hexane/THF (10:1 v/v, 55 mL). 1,6-dibromohexanes (32.7 mL, 213 mmol) was added and stirred for 2 h. The reaction was stopped with the addition of saturated NaHCO3 (50 mL) and diluted diethyl (100 mL). The organic layer was washed with water (100 mL), brine (100 mL), dried with magnesium sulfate anhydrous, treated with decolorizing charcoal, filtered and concentrated in a vacuum to give an oil with an orange color. Excess 1,6-dibromohexane was removed via vacuum distillation (0.04 torr, 55 °C) and purified by silica gel column chromatography (ethyl acetate/hexane, 1/99–5/95 v/v) to obtain an oily product. Yield: 52%. 1H NMR (Figure S2, Supplementary Material) (400 MHz, DMSO-D6) δ (ppm): 7.42–6.97, (Ha, b, c), 3.51 (Hi), 2.57 (Hd), 1.6–1.32 (He, f, g, h). FTIR (cm−1): 3062.45 (C–H aromatic), 2983 and 2912 (C–H (sp3)), 1589.22 and 1423.89 (C=C aromatic), 651.02 (C–Br).
- •
- 4-((Phenylimino)methyl)phenol (2): 4-hydroxybenzaldehyde (122 mg, 10 mmol) was added to (112 mg, 10 mmol) 2-aminobenzenethiol in 50 mL ethanol. The mixture was refluxed for 3 h. A yellow crystal was obtained after recrystallization with ethanol. Yield: 95%. 1H NMR (Figure S3, Supplementary Material) (400 MHz, DMSO-D6) δ (ppm): 10.13 (Ha), 8.46 (Hd), 7.80–6.89 (Hb, c, f). FTIR (cm−1): 3413.56 (O–H), 3100.34 (C–H aromatic), 1623.05 (C=N) 1589.45 and 1454.65.
- •
- Phenyl-(4-(6-thiophen-3-yl-hexyloxy)-benzylidene)-amine) (3): A mixture of 4-((phenylimino)methyl)phenol (1.97 g, 10 mmol), anhydrous potassium carbonate (4.14 g, 30 mmol) and 18-Crown-6 (16.6 mg, 0.1 mmol) was stirred in dried acetone (50 mL) at room temperature. Then, compound 3-(6-bromohexylthiophene) (0.81 g, 2 mmol) was added. The reaction mixture was refluxed under nitrogen with stirring for 24 h. After cooling to room temperature, the reaction mixture was poured into the saturated solution of potassium carbonate. The organic phase was collected and washed by water (3 × 100 mL), dried by anhydrous sodium sulfate and filtered. The solvent was removed by reduced pressure, and the residue was dried by vacuum to produce the crude product. Purification was accomplished by column chromatography on silica with 25% hexane in chloroform to afford the monomer [56]. Yield: 67.6%. 1H NMR (Figure S4, Supplementary Material) (400 MHz, DMSO-D6) δ (ppm): 8.5 (Hl), 7.8 (Hk), 7.4–6.9 (Hj, m, n, o), 6.6–6.9 (Ha, b, c), 3.97 (Hi), 2.67 (Hd), 1.74–1.41 (He, f, g, h). FTIR (cm−1): 2938.38, 1617, 1499.9, 1426.71, 1239.71, 1018.26.
2.3.2. Polymerization of 3TArH and Thiophene Monomers on the Surface of MNPs
2.4. Solid Phase Extraction Optimization and Reusability Studies
2.5. Analytical Performances and Real Sample Analysis
3. Results and Discussion
3.1. Characterization of the Samples
3.2. Solid Phase Extraction Optimization and Reusability Studies
3.2.1. Type of Adsorbent
3.2.2. Sample pH
3.2.3. Extraction Time
3.2.4. Desorption Studies
3.2.5. Mass of Adsorbent
3.2.6. Sample Loading Volume
3.2.7. Effect of NaCl
3.2.8. Reusability Studies
3.3. Analytical Performances and Real Sample Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franck, H.-G.; Stadelhofer, J. Production and uses of xylene derivatives. In Industrial Aromatic Chemistry; Springer: Berlin, Germany/Heidelberg, Germany, 1988; pp. 265–290. [Google Scholar]
- Jones-Lepp, T.; Gerlach, C.L.; Cooter, E.J. The power of analytical methods for measuring suspected endocrine disrupting compounds: A pilot field study. TrAC Trends Anal. Chem. 2000, 19, 286–291. [Google Scholar] [CrossRef]
- Wypych, G. Effect of plasticizers on properties of plasticized materials. In Handbook of Plasticizers; Ontario, ChemTec Publishing: Toronto, ON, Canada, 2004; pp. 193–272. [Google Scholar]
- Serôdio, P.; Nogueira, J. Considerations on ultra-trace analysis of phthalates in drinking water. Water Res. 2006, 40, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.-L.; Li, X.-Z.; Graham, N. Aqueous oxidation of dimethyl phthalate in a Fe(VI)-TiO2-UV reaction system. Water Res. 2008, 42, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S.; Karr, C.J.; Lozano, P.; Brown, E.; Calafat, A.M.; Liu, F.; Swan, S.H. Baby care products: Possible sources of infant phthalate exposure. Pediatrics 2008, 121, e260–e268. [Google Scholar] [CrossRef] [PubMed]
- PnTER, P. Hydrochemie [hydrochemistry], 4th ed.; Vydavatelstvi VSCHT: Prague, The Czech Republic, 2009. [Google Scholar]
- Ohtani, H.; Miura, I.; Ichikawa, Y. Effects of dibutyl phthalate as an environmental endocrine disruptor on gonadal sex differentiation of genetic males of the frog rana rugosa. Environ. Health Perspect. 2000, 108, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Verrotti, A.; de Felice, C. Di-2-ethylhexyl phthalate and endocrine disruption: A review. Curr. Drug Targets Immune Endocr. Metab. Disord. 2004, 4, 37–40. [Google Scholar] [CrossRef]
- Foster, P. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006, 29, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Hens, A.; Aguilar-Caballos, M. Social and economic interest in the control of phthalic acid esters. TrAC Trends Anal. Chem. 2003, 22, 847–857. [Google Scholar] [CrossRef]
- Laws of Malaysia, Food Act 1983, Food Regulations 1985. Available online: http://www.asianfoodreg.com/dynamicAssets/regulationDoc/1412157254_Malaysian-Food-Regulations-19852014.pdf (accessed on 10 March 2016).
- Ministry of Science, Technology and Innovation (MOSTI). Plastic Materials and Articles Intended to Come into Contact with Food (First Revision); Ministry of Science, Technology and Innovation (MOSTI): Cyberjaya, Malaysia, 2014. [Google Scholar]
- Amiridou, D.; Voutsa, D. Alkylphenols and phthalates in bottled waters. J. Hazard. Mater. 2011, 185, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Kohler, M.; Meierhofer, R.; Luzi, S.; Wegelin, M. Does the reuse of pet bottles during solar water disinfection pose a health risk due to the migration of plasticisers and other chemicals into the water? Water Res. 2008, 42, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Wu, C.-F.; Wu, J.-R.; Chen, B.-H.; Chen, E.K.; Chao, M.-C.; Liu, C.-K.; Ho, C.-K. The public health threat of phthalate-tainted foodstuffs in taiwan: The policies the government implemented and the lessons we learned. Environ. Int. 2012, 44, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Dauchy, X.; Chagnon, M.-C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (pet) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Leivadara, S.V.; Nikolaou, A.D.; Lekkas, T.D. Determination of organic compounds in bottled waters. Food Chem. 2008, 108, 277–286. [Google Scholar] [CrossRef]
- Liu, H.-C.; Den, W.; Chan, S.-F.; Kin, K.T. Analysis of trace contamination of phthalate esters in ultrapure water using a modified solid-phase extraction procedure and automated thermal desorption–gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1188, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Plotan, M.; Frizzell, C.; Robinson, V.; Elliott, C.T.; Connolly, L. Endocrine disruptor activity in bottled mineral and flavoured water. Food Chem. 2013, 136, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Oehlmann, J. Endocrine disruptors in bottled mineral water: Total estrogenic burden and migration from plastic bottles. Environ. Sci. Pollut. Res. 2009, 16, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chung, Y.-C. Removal of phthalate esters from aqueous solutions by chitosan bead. J. Environ. Sci. Health Part. A 2006, 41, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Julinová, M.; Slavík, R. Removal of phthalates from aqueous solution by different adsorbents: A short review. J. Environ. Manag. 2012, 94, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, C.; Liao, C.; Chang, B. Occurrence and microbial degradation of phthalate esters in taiwan river sediments. Chemosphere 2002, 49, 1295–1299. [Google Scholar] [CrossRef]
- López-Jiménez, F.J.; Rubio, S.; Pérez-Bendito, D. Determination of phthalate esters in sewage by hemimicelles-based solid-phase extraction and liquid chromatography–mass spectrometry. Anal. Chim. Acta 2005, 551, 142–149. [Google Scholar] [CrossRef]
- Farahani, H.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Screening method for phthalate esters in water using liquid-phase microextraction based on the solidification of a floating organic microdrop combined with gas chromatography–mass spectrometry. Talanta 2008, 76, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Jiang, G.-B.; Cai, Y.-Q.; Bin, H.; Wang, Y.-W.; Shen, D.-Z. Cloud point extraction coupled with HPLC-UV for the determination of phthalate esters in environmental water samples. J. Environ. Sci. 2007, 19, 874–878. [Google Scholar]
- Kerienė, I.; Maruška, A.; Sitonytė, J. Solid phase extraction and gas chromatographic-mass spectrometric analysis of phthalates in surface water: Method development and validation. Chemija 2011, 22, 204–209. [Google Scholar]
- Tahmasebi, E.; Yamini, Y. Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver (I), gold (III), copper (II) and palladium (II). Microchim. Acta 2014, 181, 543–551. [Google Scholar] [CrossRef]
- Kvistad, A.; Lundanes, E.; Greibrokk, T. Determination of alkylphenols in water samples by solid-phase extraction on to poly(styrene-divinylbenzene) and quantification by liquid chromatography with UV-detection. Chromatographia 1998, 48, 707–713. [Google Scholar] [CrossRef]
- Holadova, K.; Hajšlová, J. A comparison of different ways of sample preparation for the determination of phthalic acid esters in water and plant matrices. Int. J. Environ. Anal. Chem. 1995, 59, 43–57. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, C.-C.; Chung, Y.-C. Removal of phthalate esters by α-cyclodextrin-linked chitosan bead. Bioresour. Technol. 2007, 98, 2578–2583. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal–organic frameworks. J. Hazard. Mater. 2015, 282, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Raoov, M.; Mohamad, S.; bin Abas, M.R.; Surikumaran, H. New macroporous β-cyclodextrin functionalized ionic liquid polymer as an adsorbent for solid phase extraction with phenols. Talanta 2014, 130, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, L.; Gao, M.; Lei, H. One-pot reaction to synthesize biocompatible magnetite nanoparticles. Adv. Mater. 2005, 17, 1001–1005. [Google Scholar] [CrossRef]
- Lin, P.-C.; Yu, C.-C.; Wu, H.-T.; Lu, Y.-W.; Han, C.-L.; Su, A.-K.; Chen, Y.-J.; Lin, C.-C. A chemically functionalized magnetic nanoplatform for rapid and specific biomolecular recognition and separation. Biomacromolecules 2012, 14, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Hanif, S.; Hamid, A.; Duran, H.; Yameen, B. Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation. ACS Appl. Mater. Interf. 2013, 5, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Shahabuddin, S.; Muhamad Sarih, N.; Mohamad, S.; Joon Ching, J. SrTiO3 nanocube-doped polyaniline nanocomposites with enhanced photocatalytic degradation of methylene blue under visible light. Polymers 2016, 8, 27. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Ismail, F.H.; Shahid, M.M.; Huang, N.M. Synthesis of chitosan grafted-polyaniline/Co3O4 nanocube nanocomposites and their photocatalytic activity toward methylene blue dye degradation. RSC Adv. 2015, 5, 83857–83867. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of functionalized magnetic nanoparticles in sample preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lam, M.H.; Wu, R.S.; Jiang, B. Rapid magnetic-mediated solid-phase extraction and pre-concentration of selected endocrine disrupting chemicals in natural waters by poly (divinylbenzene-co-methacrylic acid) coated Fe3O4 core–shell magnetite microspheres for their liquid chromatography–tandem mass spectrometry determination. J. Chromatogr. A 2010, 1217, 1219–1226. [Google Scholar] [PubMed]
- Ibarra, I.S.; Miranda, J.M.; Rodriguez, J.A.; Nebot, C.; Cepeda, A. Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem. 2014, 157, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A. Fe3O4 and MnO2 assembled on halloysite nanotubes: A highly efficient solid-phase extractant for electrochemical detection of mercury(II) ions. Sens. Actuators B Chem. 2016, 228, 1–9. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.; Miranda, J.; Medina, J.; Barrado, E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion–HPLC. Talanta 2010, 80, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Rouhani, S.; Mozaffari, S. Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim. Acta 2016. [Google Scholar] [CrossRef]
- Sun, L.; Sun, X.; Du, X.; Yue, Y.; Chen, L.; Xu, H.; Zeng, Q.; Wang, H.; Ding, L. Determination of sulfonamides in soil samples based on alumina-coated magnetite nanoparticles as adsorbents. Anal. Chim. Acta 2010, 665, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; He, Q.; Yang, X.; Han, Q. Solid phase extraction and preconcentration of trace mercury (ii) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim. Acta 2010, 169, 353–360. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 2010, 81, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gómez, A.; Rubio, S. Hemimicelles of alkyl carboxylates chemisorbed onto magnetic nanoparticles: Study and application to the extraction of carcinogenic polycyclic aromatic hydrocarbons in environmental water samples. Anal. Chem. 2009, 81, 9012–9020. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, E.; Yamini, Y.; Mehdinia, A.; Rouhi, F. Polyaniline—Coated Fe3O4 nanoparticles: An anion exchange magnetic sorbent for solid—Phase extraction. J. Sep. Sci. 2012, 35, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Luo, D.; Bai, M.; Chen, Z.-W.; Feng, Y.-Q. Rapid determination of estrogens in milk samples based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 8543–8549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, Q.; Feng, Y.-Q. Dispersive microextraction based on magnetic polypyrrole nanowires for the fast determination of pesticide residues in beverage and environmental water samples. Anal. Bioanal. Chem. 2013, 405, 4765–4776. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, J. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem. Commun. 2007. [Google Scholar] [CrossRef]
- Williamson, A.W. XXII.—On etherification. Q. J. Chem. Soc. Lond. 1852, 4, 229–239. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Li, F.; Chen, L.; Yuan, K.; Yao, K.; Wang, P. Ordered microstructure induced by orientation behavior of liquid-crystal polythiophene for performance improvement of hybrid solar cells. Sol. Energy Mater. Sol. Cells 2012, 96, 266–275. [Google Scholar] [CrossRef]
- Shen, L.; Laibinis, P.E.; Hatton, T.A. Bilayer surfactant stabilized magnetic fluids: Synthesis and interactions at interfaces. Langmuir 1999, 15, 447–453. [Google Scholar] [CrossRef]
- Aydın, M.; Durmus, Z.; Kavas, H.; Esat, B.; Sözeri, H.; Baykal, A.; Yılmaz, F.; Toprak, M.S. Synthesis and characterization of poly(3-thiophene acetic acid)/Fe3O4 nanocomposite. Polyhedron 2011, 30, 1120–1126. [Google Scholar] [CrossRef]
- Vasanthi, B.J.; Ravikumar, L. Synthesis and characterization of poly(azomethine ester) s with a pendent dimethoxy benzylidene group. Open J. Polym. Chem. 2013, 3, 70–77. [Google Scholar] [CrossRef]
- Cótica, L.F.; Santos, I.A.; Girotto, E.M.; Ferri, E.V.; Coelho, A.A. Surface spin disorder effects in magnetite and poly(thiophene)-coated magnetite nanoparticles. J. Appl. Phys. 2010, 108, 064325. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.Y. Stimuli—Responsive controlled—Release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Jayabharathi, J.; Ramanathan, P.; Thanikachalam, V.; Karunakaran, C. Optical and theoretical studies on Fe3O4–imidazole nanocomposite and clusters. New J. Chem. 2015, 39, 3801–3812. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.F.; Yang, M.; Wu, X.F.; Tian, Y.J. Key engineering materials. In Synthesis of Low Agglomerating Spherical α-Fe2O3 Nanopowders; Trans Tech Publisher: Pfaffikon, Switzerland, 2008; pp. 1568–1569. [Google Scholar]
- Darab, J.G.; Linehan, J.C.; Matson, D.W. Effect of agglomerate size on the catalytic activity of an iron oxyhydroxide nanocrystalline powder toward carbon–carbon bond scission in naphthylbibenzylmethane. Energy Fuels 1994, 8, 1004–1005. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Y.; Liu, H. Synthesis and characterization of micron—Sized monodisperse superparamagnetic polymer particles with amino groups. J. Polym. Sci. Part A 2005, 43, 3433–3439. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rao, N.C.; Prasad, K.K.; Karthikeyan, J. Treatment of simulated reactive yellow 22 (Azo) dye effluents using spirogyra species. Waste Manag. 2002, 22, 575–582. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, H. Adsorption of di-n-butyl phthalate onto nutshell-based activated carbon. Equilibrium, kinetics and thermodynamics. Adsorpt. Sci. Technol. 2009, 27, 685–700. [Google Scholar] [CrossRef]
- Miskam, M.; Bakar, N.K.A.; Mohamad, S. Determination of polar aromatic amines using newly synthesized sol–gel titanium(IV) butoxide cyanopropyltriethoxysilane as solid phase extraction sorbent. Talanta 2014, 120, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.G. Organic Chemistry; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Tahmasebi, E.; Yamini, Y.; Moradi, M.; Esrafili, A. Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: Synthesis and application as a new sorbent for solid-phase extraction. Anal. Chim. Acta 2013, 770, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Le Zhang, X.; Niu, H.Y.; Zhang, S.X.; Cai, Y.Q. Preparation of a chitosan-coated C18-functionalized magnetite nanoparticle sorbent for extraction of phthalate ester compounds from environmental water samples. Anal. Bioanal. Chem. 2010, 397, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, A.; Toghroli, M.; Kamankesh, M. Zeolite/Fe3O4 as a new sorbent in magnetic solid—Phase extraction followed by gas chromatography for determining phthalates in aqueous samples. J. Sep. Sci. 2015, 38, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.; Chen, G.; Zhang, W.; Cai, Y.; Kong, R.; Wang, X.; Suo, Y.; You, J. Determination of phthalate esters in environmental water by magnetic zeolitic imidazolate framework-8 solid-phase extraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2015, 1409, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Staples, C. Phthalate Esters; Springer Science and Business Media: Berlin, Germany, 2003. [Google Scholar]











| Sample | Pore size (nm) | Surface area (m2·g−1) |
|---|---|---|
| MNP | 20.2 | 37.37 |
| MNP@PTh | 18.3 | 95.6 |
| MNP@P3TArH | 12.09 | 103.80 |
| Analytes | R2 | Linear range (µg·L−1) | LOD (µg·L−1) | LOQ (µg·L−1) | RSD (%) Interday n = 3 | RSD (%) Intraday n = 7 |
|---|---|---|---|---|---|---|
| DMP | 0.992 | 0.5–50 | 0.462 | 1.539 | 3.4 | 4.8 |
| DEP | 0.992 | 0.5–50 | 0.468 | 1.562 | 5.0 | 4.3 |
| DPP | 0.997 | 0.5–50 | 0.286 | 0.954 | 4.6 | 3.7 |
| DBP | 0.998 | 0.1–50 | 0.063 | 0.213 | 4.5 | 4.5 |
| BBP | 0.996 | 0.1–50 | 0.080 | 0.268 | 4.8 | 4.3 |
| DCP | 0.993 | 0.5–50 | 0.332 | 1.106 | 4.7 | 4.0 |
| DEHP | 0.997 | 0.1–50 | 0.054 | 0.182 | 3.0 | 4.0 |
| DNOP | 0.997 | 0.1–50 | 0.073 | 0.244 | 3.6 | 4.9 |
| Analyte | Method | LOD (µg·L−1) | LDR (µg·L−1) | RSD (%) | Reference |
|---|---|---|---|---|---|
| DBP, DEHP, DOA | MNP@PTh-GC-FID | 0.2–0.4 | 0.4–100 | 4–12.3 | [72] |
| DPP, DBP, DCP, DNOP | MNP@Chitosan-C18-HPLC-UV | 0.012–0.037 | 0.001–0.01 | 2.1–6.8 | [73] |
| DBP, DEHP | MNP@Zeolite-GC-FID | 2.80–3.2 | 10–1200 | 10%–13% | [74] |
| DMP, DEP, DBP, BBP, DNOP | MNP@ZIF-8-HPLC | 0.08–0.24 | 1–100 | <5.5 | [75] |
| DMP, DPP, DEP, DBP, BBP, DCP, DEHP, DNOP | MNP@P3TArH- GC-FID | 0.05–0.09 | 0.1–50 | 3.0–5.0 | This study |
| Analyte | MNP@P3TArH MSPE (±RSD%, n = 3) | |
|---|---|---|
| Mineral water | Commercial fresh milk | |
| DMP | 85(5.8) | 68(5.0) |
| DEP | 85 (4.9) | 67(3.0) |
| DPP | 88(1.3) | 72(7.7) |
| DBP | 95(2.4) | 85(3.3) |
| BBP | 93(3.0) | 82(3.8) |
| DCP | 90(4.7) | 77(5.8) |
| DEHP | 99(1.3) | 89(4.5) |
| DNOP | 101(4.2) | 91(3.3) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baharin, S.N.A.; Muhamad Sarih, N.; Mohamad, S. Novel Functionalized Polythiophene-Coated Fe3O4 Nanoparticles for Magnetic Solid-Phase Extraction of Phthalates. Polymers 2016, 8, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8050117
Baharin SNA, Muhamad Sarih N, Mohamad S. Novel Functionalized Polythiophene-Coated Fe3O4 Nanoparticles for Magnetic Solid-Phase Extraction of Phthalates. Polymers. 2016; 8(5):117. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8050117
Chicago/Turabian StyleBaharin, Siti Nor Atika, Norazilawati Muhamad Sarih, and Sharifah Mohamad. 2016. "Novel Functionalized Polythiophene-Coated Fe3O4 Nanoparticles for Magnetic Solid-Phase Extraction of Phthalates" Polymers 8, no. 5: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8050117