Methacrylate-Based Copolymers for Polymer Optical Fibers
Abstract
:1. Introduction
- significantly less material requirements,
- short fibers can be produced in a rapid and efficient manner,
- process variations can be implemented from run to run.
2. Materials and Methods
2.1. General Information
2.2. Copolymer Preparation
2.2.1. Precipitated Copolymers
2.2.2. Solid Copolymer Samples
2.2.3. Polymer Fiber Production
3. Results and Discussion
3.1. Monomer Selection and Experimental Setup
3.2. Copolymerization Parameters
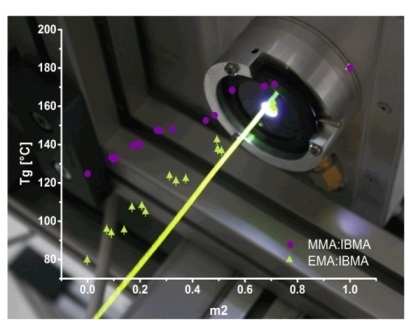
3.3. Thermo-Chemical Behavior
3.4. Application Case HT-POF
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Zettabyte Era: Trends and Analysis. Available online: http://www.cisco.com/c/en/us/solutions/service-provider/visual-networking-index-vni/white-paper-listing.html (accessed on 28 November 2016).
- Koutitas, G.; Demestichas, P. A review of energy efficiency in telecommunication networks. Telfor J. 2010, 2, 2–7. [Google Scholar]
- Ciordia, O.; Esteban, C.; Pardo, C.; Pérez de Aranda, R. Commercial silicon for gigabit communication over SI-POF. In Proceedings of the 22nd International Conference on Plastic Optical Fibers, POF 2013, Búzios, Brazil, 11–13 September 2013; Volume 22, pp. 109–116.
- Reviriego, P.; Perez-Aranda, R.; Pardo, C. Introducing energy efficiency in the VDE 0885-763 standard for high speed communication over plastic optical fibers. IEEE Commun. Mag. 2013, 51, 97–102. [Google Scholar] [CrossRef]
- Agrawal, G.P. Fiber-Optic Communication Systems, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 23–28. [Google Scholar]
- Koike, Y.; Ishigure, T.; Nihei, E. High-bandwidth graded-index polymer optical fiber. J. Lightw. Technol. 1995, 13, 1475–1489. [Google Scholar] [CrossRef]
- Koike, Y.; Asai, M. The future of plastic optical fiber. NPG Asia Mater. 2009, 1, 22–28. [Google Scholar] [CrossRef]
- Giaretta, G.; White, W.; Wegmuller, M.; Onishi, T. High-speed (11 Gbit/s) data transmission using perfluorinated graded-index polymer optical fibers for short interconnects (<100 m). IEEE Photonics Technol. Lett. 2000, 12, 347–349. [Google Scholar]
- White, W. Graded-index POF in active optical cables. In Proceedings of the 24th International Conference on Plastic Optical Fibers, POF 2015, Nuremberg, Germany, 11–13 September 2015; Volume 24, p. 312.
- Van den Boom, H.P.A.; Li, W.; van Bennekom, P.K.; Tafur Monroy, I.; Giok-Djan, K. High-capacity transmission over polymer optical fiber. IEEE J. Sel. Top. Quantum Electron. 2001, 7, 461–470. [Google Scholar] [CrossRef]
- Schlepple, N.; Nishigaki, M.; Uemura, H.; Furuyama, H.; Sugizaki, Y.; Shibata, H.; Koike, Y. Ultracompact 4 × 3.4 Gbps optoelectronic package for an active optical HDMI cable. IEEE CPMT Symp. Jpn. 2012, 1–4. [Google Scholar] [CrossRef]
- Takizuka, H.; Torikai, T.; Mitsui, A.; Suzuki, H.; Watanabe, Y.; Toma, T.; Koike, Y. A proposal of novel optical interface to transmit 8K-UHDTV for consumer applications. In Proceedings of the 18th Microoptics Conference, MOC’13, Tokyo, Japan, 27–30 October 2013; p. 14043816.
- Toma, T.; Takizuka, H.; Torikai, T.; Suzuki, H.; Ogi, T.; Koike, Y. Development of a household high-definition video transmission system based on ballpoint-pen technology. Synthesiology 2014, 7, 118–128. [Google Scholar] [CrossRef]
- Beckers, M.; Schlüter, T.; Vad, T.; Gries, T.; Bunge, C.-A. An overview on fabrication methods for polymer optical fibers. Polym. Int. 2015, 64, 25–36. [Google Scholar] [CrossRef]
- Zubia, J.; Arrue, J. Plastic optical fibers: An introduction to their technological processes and applications. Opt. Fiber Technol. 2001, 7, 101–140. [Google Scholar] [CrossRef]
- Kuzyk, M.G. Polymer Fiber Optics: Materials, Physics, and Applications; CRC: Boca Raton, FL, USA, 2007; pp. 53–68. [Google Scholar]
- Daum, W. Reliability of polymer optical fibres. In Proceedings of the 27th European Conference on Optical Communication, ECOC’01, Amsterdam, The Netherlands, 30 September–4 October 2001; pp. 68–69.
- Flipsen, T.A.C.; Steendam, R.; Pennings, A.J.; Hadziioannou, G. A novel thermoset polymer optical fiber. Adv. Mater. 1996, 8, 45–48. [Google Scholar] [CrossRef]
- Peters, K. Polymer optical fiber sensors—A review. Smart Mater. Struct. 2011, 20, 17. [Google Scholar] [CrossRef]
- Bilro, L.; Alberto, N.; Pinto, J.L.; Nogueira, R. Optical sensors based on plastic fibers. Sensors 2012, 12, 12184–12207. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.D.; Chu, P.L.; Xiong, Z.; Whitbread, T.W.; Chaplin, R.P. Dye-doped step-index polymer optical fiber for broadband optical amplification. J. Lightw. Technol. 1996, 14, 2215–2223. [Google Scholar] [CrossRef]
- Kondo, A.; Araki, T.; Koike, Y. Binary amorphous copolymers based graded index polymer optical fiber. In Proceedings of the 21st International Conference on Plastic Optical Fibers, POF 2012, Atlanta, GA, USA, 10–12 September 2012; Volume 21, pp. 74–77.
- Koike, K.; Mikeš, F.; Okamoto, Y.; Koike, Y. Design, synthesis, and characterization of a partially chlorinated acrylic copolymer for low-loss and thermally stable graded index plastic optical fibers. J. Polym. Sci. A 2009, 47, 3352–3361. [Google Scholar] [CrossRef]
- Zaremba, D.; Evert, R.; Cichosch, A.; Caspary, R.; Kowalsky, W.; Johannes, H.-H. Novel concepts for copolymer based high temperature POFs. In Proceedings of the 23rd International Conference on Plastic Optical Fibers, POF 2014, Yokohama, Japan, 8–10 October 2014; Volume 23, pp. 69–73.
- Möhl, S.; Cichosch, A.; Schütz, S.; Caspary, R.; Kowalsky, W.; Johannes, H.-H. Materials for polymer optical fiber amplifiers. In Proceedings of the 21st International Conference on Plastic Optical Fibers, POF 2012, Atlanta, GA, USA, 10–12 September 2012; Volume 21, pp. 50–55.
- Koike, Y.; Tanio, N.; Ohtsuka, Y. Light scattering and heterogeneities in low-loss poly(methy-methacrylate) glasses. Macromolecules 1989, 22, 1367–1373. [Google Scholar] [CrossRef]
- Zaremba, D.; Evert, R. Materials, chemical properties and analysis. In Polymer Optical Fibres; Woodhead Publishing: Duxford, UK, 2017; pp. 153–186. [Google Scholar]
- Tieke, B. Makromolekulare Chemie, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 146–163. [Google Scholar]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Kelen, T.; Tüdös, F. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J. Macromol. Sci. Part A 1975, 1, 1–27. [Google Scholar] [CrossRef]
- Alfrey, T.; Price, C.C. Relative reactivities in vinyl copolymerization. J. Polym. Sci. 1947, 2, 101–106. [Google Scholar] [CrossRef]
- Schütz, S. Optische Spezialfasern aus Polymeren und Fluoridglas; Dr. Hut: München, Germany, 2013; pp. 35–44. [Google Scholar]
- Koike, Y. Fundamentals of Plastic Optical Fibers; Wiley-VCH: Weinheim, Germany, 2015; pp. 84–86. [Google Scholar]
- Polymer Handbook; Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. (Eds.) Wiley: New York, NY, USA, 1989.
- Greenley, R.Z. An expanded listing of revised Q and e values. J. Macromol. Sci. Part A 1980, 14, 427–443. [Google Scholar] [CrossRef]
- Fasla, A.; Ould Kada, S.; Seghier, Z.; Périchaud, A. Cyclohexyl methacrylate with tetra (ethylene glycol) dimethacrylate in tertahydrofuran radical copolymerization. Mater. Sci. Forum 2009, 609, 63–67. [Google Scholar] [CrossRef]
- Imoto, M.; Otsu, T.; Tsuda, K.; Ito, T. Vinyl Polymerization. LXXIII. Polymerization and copolymerization of bornyl or isobornyl methacrylate. J. Polym. Sci. A 1964, 2, 1407–1419. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakatsuka, S.; Iwafuji, T.; Kuriki, K.; Imai, N.; Nakamoto, T.; Claude, C.D.; Sasaki, K.; Koike, Y.; Okamoto, Y. Fabrication and superfluorescence of rare-earth chelate-doped graded index polymer optical fibers. Appl. Phys. Lett. 1997, 71, 2421. [Google Scholar] [CrossRef]
- Yu, J.; Tao, X.; Tam, H. Fabrication of UV sensitive single-mode polymeric optical fiber. Opt. Mater. 2006, 28, 181–188. [Google Scholar] [CrossRef]
- Cichosch, A. Entwicklung von Materialien für den Einsatz in Polymer Optischen Faserverstärkern; Dr. Hut: München, Germany, 2015; pp. 85–86. [Google Scholar]
- Mayo, F.R.; Lewis, F.M. Copolymerization, I. A basis for comparing the behavior of monomers in copolymerization; the copolymerization of styrene and methyl methacrylate. J. Am. Chem. Soc. 1944, 66, 1594–1601. [Google Scholar] [CrossRef]
- Ziemann, O.; Krauser, J.; Zamzow, P.E.; Daum, W. POF-Handbuch: Optische Kurzstrecken-Übertragungssysteme (German Edition); Springer: Dordrecht, The Netherlands, 2007; pp. 79–90. [Google Scholar]
- Yang, D.; Yu, J.; Tao, X.; Tam, H. Structural and mechanical properties of polymeric optical fiber. Mater. Sci. Eng. A 2004, 364, 256–259. [Google Scholar] [CrossRef]
- Graf, J. Entwicklung und Untersuchungen zur Herstellung Verlustarmer Passiver Wellenleiter und Verstärkender Wellenleiter; Universitätsbibliothek Universität des Saarlandes: Saarbrücken, Germany, 1999; pp. 27–32. [Google Scholar]
- Strobel, O.; Rejeb, R.; Lubkoll, J. Communication in automotive systems: Principles, limits and new trends for vehicles, airplanes and vessels. In Proceedings of the 12th International Conference on Transparent Optical Networks, ICTON 2010, Munich, Germany, 27 June–1 July 2010; pp. 1–6.
- Seibl, D.; Bohm, M.; Strobel, O. Polymer-optical-fiber data bus technologies for MOST applications in vehicles. In Proceedings of the 2nd ICTON Mediterranean Winter, Marrakech, Morocco, 11–13 December 2008; Volume 2, pp. 1–6.
- Zeeb, E. Optical communication, optical data bus systems in cars: current status and future challenges. In Proceedings of the 27th European Conference on Optical Communication, ECOC’01, Amsterdam, The Netherlands, 30 September–4 October 2001; pp. 70–71.






| Copolymer | Copolymer Parameter (AP) a | Copolymer Parameter (FR) b | Copolymer Parameter (KT) c | Tg Trend |
|---|---|---|---|---|
| MMA:EMA d | r1 = 0.888 | r1 = 0.680 | r1 = 0.753 | – |
| r2 = 1.068 | r2 = 0.777 | r2 = 0.912 | ||
| MMA:PrMA | r1 = 1.157 | r1 = 0.758 | r1 = 0.761 | – |
| r2 = 0.863 | r2 = 1.080 | r2 = 1.090 | ||
| MMA:n-ButMA | r1 = 0.857 | r1 = 0.694 | r1 = 0.733 | – |
| r2 = 1.148 | r2 = 1.047 | r2 = 1.145 | ||
| MMA:t-ButMA | r1 = 0.913 | r1 = 0.641 | r1 = 0.685 | + |
| r2 = 1.067 | r2 = 1.468 | r2 = 1.605 | ||
| MMA:CHMA | r1 = 0.954 | r1 = 0.925 | r1 = 0.933 | o |
| r2 = 1.007 | r2 = 1.170 | r2 = 1.201 | ||
| MMA:BzMA | r1 = 0.824 | r1 = 0.447 | r1 = 0.475 | – |
| r2 = 1.210 | r2 = 1.261 | r2 = 1.373 | ||
| MMA:IBMA | r1 = 1.376 | r1 = 0.807 | r1 = 0.883 | + |
| r2 = 0.727 | r2 = 0.546 | r2 = 0.765 | ||
| EMA:MMA d | r1 = 1.068 | r1 = 0.777 | r1 = 0.912 | + |
| r2 = 0.888 | r2 = 0.680 | r2 = 0.753 | ||
| EMA:PrMA | r1 = 1.077 | r1 = 1.634 | r1 = 1.552 | – |
| r2 = 0.922 | r2 = 1.088 | r2 = 1.005 | ||
| EMA:n-ButMA | r1 = 0.782 | r1 = 0.292 | r1 = 0.339 | – |
| r2 = 1.202 | r2 = 0.690 | r2 = 0.828 | ||
| EMA:t-ButMA | r1 = 0.830 | r1 = 1.145 | r1 = 1.106 | + |
| r2 = 1.113 | r2 = 1.544 | r2 = 1.481 | ||
| EMA:CHMA | r1 = 0.906 | r1 = 0.659 | r1 = 0.727 | + |
| r2 = 1.097 | r2 = 0.973 | r2 = 1.159 | ||
| EMA:BzMA | r1 = 0.759 | r1 = 0.587 | r1 = 0.728 | – |
| r2 = 1.279 | r2 = 0.976 | r2 = 1.366 | ||
| EMA:IBMA | r1 = 1.277 | r1 = 0.786 | r1 = 1.000 | + |
| r2 = 0.774 | r2 = 0.269 | r2 = 0.811 |
| Fiber | Tensile Strength (N/mm²) | Attenuation (dB/m) | Tg (°C) | Decomposition Onset a (°C) |
|---|---|---|---|---|
| PMMA (self-made) | 83 | 1–2 | 124 | 93 |
| MMA:IBMA (33.4 mol %) | 72 | 2–3 | 135 | 251 |
| EMA:IBMA (50.0 mol %) | 43 | 08–12 | 125 | 234 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaremba, D.; Evert, R.; Neumann, L.; Caspary, R.; Kowalsky, W.; Menzel, H.; Johannes, H.-H. Methacrylate-Based Copolymers for Polymer Optical Fibers. Polymers 2017, 9, 34. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9020034
Zaremba D, Evert R, Neumann L, Caspary R, Kowalsky W, Menzel H, Johannes H-H. Methacrylate-Based Copolymers for Polymer Optical Fibers. Polymers. 2017; 9(2):34. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9020034
Chicago/Turabian StyleZaremba, Daniel, Robert Evert, Laurie Neumann, Reinhard Caspary, Wolfgang Kowalsky, Henning Menzel, and Hans-Hermann Johannes. 2017. "Methacrylate-Based Copolymers for Polymer Optical Fibers" Polymers 9, no. 2: 34. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9020034