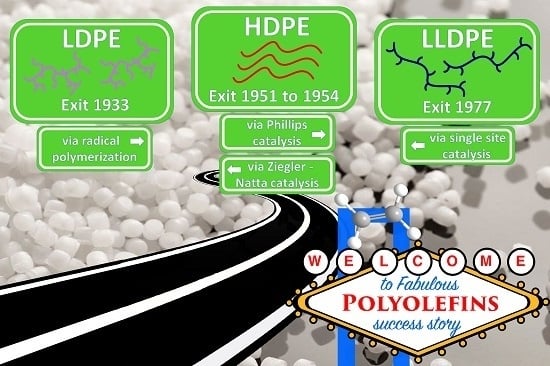
Polyolefins, a Success Story
Abstract
:1. Introduction
2. Polyolefins, the Beginning of the Story: How Does It All Start?
3. Single-Center Technology
4. Developments in Polyolefins Driven by Molecular Catalysts
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Severn, J.R.; Chadwick, J.C. (Eds.) Tailor-Made Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- AlMa’adeed, M.A.-A.; Krupa, I. Introduction. In Polyolefin Compounds and Materials; AlMa’adeed, M.A.-A., Krupa, I., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–11. [Google Scholar]
- Hutley, T.J.; Ouederni, M. Polyolefins—The History and Economic Impact. In Polyolefin Compounds and Materials; AlMa’adeed, M.A.-A., Krupa, I., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 13–50. [Google Scholar]
- Stalzer, M.M.; Delferro, M.; Marks, T.J. Supported Single-Site Organometallic Catalysts for the Synthesis of High-Performance Polyolefins. Catal. Lett. 2014, 145, 3–14. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef] [PubMed]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Pechmann, H.V. Ueber Diazomethan und Nitrosoacylamine. Berichte der Deutschen Chemischen Gesellschaft 1898, 31, 2640–2646. [Google Scholar] [CrossRef]
- Staudinger, H. Über Polymerisation. Berichte der Deutschen Chemischen Gesellschaft B 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Staudinger, H.; Fritschi, J. Über Isopren und Kautschuk. 5. Mitteilung. Über die Hydrierung des Kautschuks und über seine Konstitution. Helv. Chim. Acta 1922, 5, 785–806. [Google Scholar] [CrossRef]
- Staudinger, H. Über die Konstitution des Kautschuks (6. Mitteilung). Berichte der Deutschen Chemischen Gesellschaft B 1924, 57, 1203–1208. [Google Scholar] [CrossRef]
- Seymour, R.B.; Cheng, T. (Eds.) History of Polyolefins; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Utracki, L.A. Polymer Blends; iSmithers Rapra Publishing: Shawbury, UK, 2000. [Google Scholar]
- Seymour, R.B.; Cheng, T. (Eds.) Advances in Polyolefins; Springer: Boston, MA, USA, 1987. [Google Scholar]
- Fawcett, E.W.; Gibson, R.O. Improvements in or Relating to the Polymerisation of Ethylene. Patent GB471590, 6 September 1937. [Google Scholar]
- McMillan, F.M. The Chain Straighteners: Fruitful Innovation; the Discovery of Linear and Stereoregular Synthetic Polymers; Macmillan: New York, NY, USA, 1979. [Google Scholar]
- Fischer, M.D. Verfahren zur Herstellung von festen Polymerisaten aus AEthylen oder aethylenreichenGasen. Patent DE874215 C, 20 April 1953. [Google Scholar]
- Bailey, G.C.; Reid, J.A. Catalytic Polymerization of Olefins. U.S. Patent 2381198, 7 August 1945. [Google Scholar]
- Bailey, G.C.; Reid, J.A. SiO2-Al2O3-NiO Catalyst and Its Preparation. U.S. Patent 2581228, 1 January 1952. [Google Scholar]
- Bailey, G.C.; Reid, J.A. Catalytic Polymerization of Olefins. U.S. Patent 2606940, 12 August 1952. [Google Scholar]
- Sailors, H.R.; Hogan, J.P. History of Polyolefins. J. Macromol. Sci. Part. Chem. 1981, 15, 1377–1402. [Google Scholar] [CrossRef]
- Hogan, J.P.; Banks, R.L. Polymers and Production Thereof. U.S. Patent 2825721, 4 March 1958. [Google Scholar]
- Hogan, J.P.; Banks, R.L. Improvements in or Relating to Polymerization of Olefins. Patent GB790195, 10 October 1957. [Google Scholar]
- Hogan, J.P.; Banks, R.L. Perfectionnements aux Polymères et à Leur Production par Action Catalytique. Patent FR1144587, 15 October 1957. [Google Scholar]
- Hogan, J.P. Ethylene polymerization catalysis over chromium oxide. J. Polym. Sci. 1970, 8, 2637–2652. [Google Scholar] [CrossRef]
- Hogan, J.P. Ch.6—Catalysis of the Phillips Petroleum Company Polyethylene Process. In Applied Industrial Catalysis; Academic Press: New York, NY, USA, 1983; pp. 149–176. [Google Scholar]
- McDaniel, M.P. Supported Chromium Catalysts for Ethylene Polymerization. In Advances in Catalysis; Academic Press: New York, NY, USA, 1985; Volume 33, pp. 47–98. [Google Scholar]
- McDaniel, M.P.; Rohlfing, D.C.; Benham, E.A. Long Chain Branching in Polyethylene from the Phillips Chromium Catalyst. Polym. React. Eng. 2003, 11, 101–132. [Google Scholar] [CrossRef]
- Janzen, J.; Colby, R.H. Diagnosing long-chain branching in polyethylenes. J. Mol. Struct. 1999, 485–486, 569–584. [Google Scholar] [CrossRef]
- Ziegler, K.; Bähr, K. Über den vermutlichen Mechanismus der Polymerisationen durch Alkalimetalle (Vorläufige Mitteilung). Berichte der Deutschen Chemischen Gesellschaft B 1928, 61, 253–263. [Google Scholar] [CrossRef]
- Ziegler, K. A Forty Years’ Stroll through the Realms of Organometallic Chemistry. In Advances in Organometallic Chemistry; Academic Press: New York, NY, USA, 1968; Volume 6, pp. 1–17. [Google Scholar]
- Ziegler, K.; Heinz, B.; Erhard, H.; Heinz, M. High Molecular Polyethylenes. Patent DE973626, 14 April 1960. [Google Scholar]
- Ziegler, K.; Breil, H.; Martin, H. High molecular polyethylenes. Patent GB799392, 21 March 1957. [Google Scholar]
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline high polymers of α-olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Natta, G.; Pino, P.; Mazzanti, G. Prevailingly to Substantially Atactic Crude Polymers and Methods for Producing the Same. U.S. Patent 3261820, 19 July 1966. [Google Scholar]
- Natta, G.; Pasquon, I. Process for Polymerizing Unsaturated Hydrocarbons to Crystalline Polymers of Regulated Molecular Weight. U.S. Patent 3245973, 12 April 1966. [Google Scholar]
- Natta, G.; Crespi, G. Block Polymers of Alpha-Olefines, Processes for Producing the Same, and Mixtures Thereof with Isotactic Polyolefines. U.S. Patent 3175999, 30 March 1965. [Google Scholar]
- Natta, G.; Pino, P.; Mazzanti, G. Isotactic Polypropylene. U.S. Patent 3112300, 26 November 1963. [Google Scholar]
- Corradini, P. The impact of the discovery of stereoregular polymers in macromolecular science. Macromol. Symp. 1995, 89, 1–11. [Google Scholar] [CrossRef]
- Galli, P. Forty years of industrial developments in the field of isotactic polyolefins. Macromol. Symp. 1995, 89, 13–26. [Google Scholar] [CrossRef]
- The Nobel Prize in Chemistry 1963. Available online: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1963/ (accessed on 15 September 2015).
- Martin, H. Polymers, Patents, Profits: A Classic Case Study for Patent Infighting; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Kaminsky, W. Discovery of Methylaluminoxane as Cocatalyst for Olefin Polymerization. Macromolecules 2012, 45, 3289–3297. [Google Scholar] [CrossRef]
- Sinn, H.; Hinck, H.; Bandermann, F.; Grützmacher, H.F. Bildung von Poly(methyl-methylen-aluminium). Angew. Chem. 1968, 80, 190. [Google Scholar] [CrossRef]
- Kaminsky, W.; Vollmer, H.-J.; Heins, E.; Sinn, H. Die Bildung von Dimetalloalkylenen, eine unvermeidliche Nebenreaktion homogener ZIiegler-Katalysatoren. Makromol. Chem. 1974, 175, 443–456. [Google Scholar] [CrossRef]
- Andresen, A.; Cordes, H.-G.; Herwig, J.; Kaminsky, W.; Merck, A.; Mottweiler, R.; Pein, J.; Sinn, H.; Vollmer, H.-J. Halogen-Free Soluble Ziegler Catalysts for the Polymerization of Ethylene. Control of Molecular Weight by Choice of Temperature. Angew. Chem. Int. Ed. Engl. 1976, 15, 630–632. [Google Scholar] [CrossRef]
- Imhoff, D.W.; Simeral, L.S.; Sangokoya, S.A.; Peel, J.H. Characterization of Methylaluminoxanes and Determination of Trimethylaluminum Using Proton NMR. Organometallics 1998, 17, 1941–1945. [Google Scholar] [CrossRef]
- Ehm, C.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V. Role(s) of TMA in polymerization. Dalton Trans. 2016, 45, 6847–6855. [Google Scholar] [CrossRef] [PubMed]
- Ghiotto, F.; Pateraki, C.; Tanskanen, J.; Severn, J.R.; Luehmann, N.; Kusmin, A.; Stellbrink, J.; Linnolahti, M.; Bochmann, M. Probing the Structure of Methylalumoxane (MAO) by a Combined Chemical, Spectroscopic, Neutron Scattering, and Computational Approach. Organometallics 2013, 32, 3354–3362. [Google Scholar] [CrossRef]
- Hirvi, J.T.; Bochmann, M.; Severn, J.R.; Linnolahti, M. Formation of Octameric Methylaluminoxanes by Hydrolysis of Trimethylaluminum and the Mechanisms of Catalyst Activation in Single-Site α-Olefin Polymerization Catalysis. ChemPhysChem 2014, 15, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Jordan, R.F.; Dasher, W.E.; Echols, S.F. Reactive cationic dicyclopentadienyl zirconium(IV) complexes. J. Am. Chem. Soc. 1986, 108, 1718–1719. [Google Scholar] [CrossRef]
- Jordan, R.F.; Bajgur, C.S.; Willett, R.; Scott, B. Ethylene polymerization by a cationic dicyclopentadienyl zirconium(IV) alkyl complex. J. Am. Chem. Soc. 1986, 108, 7410–7411. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do New Century Catalysts Unravel the Mechanism of Stereocontrol of Old Ziegler—Natta Catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Ewen, J.A. Mechanisms of stereochemical control in propylene polymerizations with soluble Group 4B metallocene/methylalumoxane catalysts. J. Am. Chem. Soc. 1984, 106, 6355–6364. [Google Scholar] [CrossRef]
- Kaminsky, W.; Külper, K.; Brintzinger, H.H.; Wild, F.R.W.P. Polymerisation von Propen und Buten mit einem chiralen Zirconocen und Methylaluminoxan als Cokatalysator. Angew. Chem. 1985, 97, 507–508. [Google Scholar] [CrossRef]
- Ewen, J.A.; Jones, R.L.; Razavi, A.; Ferrara, J.D. Syndiospecific propylene polymerizations with Group IVB metallocenes. J. Am. Chem. Soc. 1988, 110, 6255–6256. [Google Scholar] [CrossRef] [PubMed]
- Ewen, J.A.; Elder, M.J.; Jones, R.L.; Haspeslagh, L.; Atwood, J.L.; Bott, S.G.; Robinson, K. Metallocene/polypropylene structural relationships: Implications on polymerization and stereochemical control mechanisms. Makromol. Chem. Macromol. Symp. 1991, 48–49, 253–295. [Google Scholar] [CrossRef]
- Coates, G.W.; Waymouth, R.M. Oscillating Stereocontrol: A Strategy for the Synthesis of Thermoplastic Elastomeric Polypropylene. Science 1995, 267, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Wild, F.R.W.P.; Zsolnai, L.; Huttner, G.; Brintzinger, H.H. Ansa-Metallocene Derivatives: IV. Synthesis and molecular structures of chiral ansa-titanocene derivatives with bridged tetrahydroindenyl ligands. J. Organomet. Chem. 1982, 232, 233–247. [Google Scholar] [CrossRef]
- Schnutenhaus, H.; Brintzinger, H.H. 1,1’-Trimethylenebis(η5−3-tert-butylcyclopentadienyl)-titanium(IV)Dichloride, a Chiral ansa-Titanocene Derivative. Angew. Chem. Int. Ed. Engl. 1979, 18, 777–778. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Ewen, J.A. Symmetry rules and reaction mechanisms of Ziegler–Natta catalysts1. J. Mol. Catal. Chem. 1998, 128, 103–109. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, P.J.; Bunel, E.; Schaefer, W.P.; Bercaw, J.E. Scandium complex [{(η5-C5Me4)Me2Si(η1-NCMe3)}(PMe3)ScH]2: A unique example of a single-component α-olefin polymerization catalyst. Organometallics 1990, 9, 867–869. [Google Scholar] [CrossRef]
- Gielens, E.E.; Tiesnitsch, J.Y.; Hessen, B.; Teuben, J.H. Titanium Hydrocarbyl Complexes with a Linked Cyclopentadienyl—Alkoxide Ancillary Ligand; Participation of the Ligand in an Unusual Activation of a (Trimethylsilyl)methyl Group. Organometallics 1998, 17, 1652–1654. [Google Scholar] [CrossRef]
- Piers, W.E.; Shapiro, P.J.; Bunel, E.E.; Bercaw, J.E. Coping with Extreme Lewis Acidity: Strategies for the Synthesis of Stable, Mononuclear Organometallic Derivatives of Scandium. Synlett 1990, 1990, 74–84. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Fu, P.-F.; Stern, C.L.; Marks, T.J. A Novel Phenolate “Constrained Geometry” Catalyst System. Efficient Synthesis, Structural Characterization, and α-Olefin Polymerization Catalysis. Organometallics 1997, 16, 5958–5963. [Google Scholar] [CrossRef]
- Cano, J.; Kunz, K. How to synthesize a constrained geometry catalyst (CGC)—A survey. J. Organomet. Chem. 2007, 692, 4411–4423. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Babinec Susan, S.; Blanchard, M.; Guest, M.J.; Walther, B.; Chaudhary, B.I.; Barry, R.P. Compositions of Interpolymers of Alpha-Olefin Monomers with One or More Vinyl or Vinylidene Aromatic Monomers. European Patent WO9920685, 2 August 2000. [Google Scholar]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Soares, J.B.P.; McKenna, T.F.L. Polyolefin Reaction Engineering; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Zuccaccia, C.; Macchioni, A.; Busico, V.; Cipullo, R.; Talarico, G.; Alfano, F.; Boone, H.W.; Frazier, K.A.; Hustad, P.D.; Stevens, J.C.; et al. Intra- and Intermolecular NMR Studies on the Activation of Arylcyclometallated Hafnium Pyridyl-Amido Olefin Polymerization Precatalysts. J. Am. Chem. Soc. 2008, 130, 10354–10368. [Google Scholar] [CrossRef] [PubMed]
- Heurtefeu, B.; Vaultier, F.; Leino, R.; Boisson, C.; Cramail, H. Single-Site Catalysts. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2014; Volume 12, pp. 551–602. [Google Scholar]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional Catalysts for Isotactic Propene Polymerization in Solution Developed by Using High-Throughput-Screening Technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Kashiwa, N.; Fujita, T. FI Catalysts: A New Family of High Performance Catalysts for Olefin Polymerization. Adv. Synth. Catal. 2002, 344, 477–493. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, M. The Chemistry of Catalyst Activation: The Case of Group 4 Polymerization Catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: I. Tris(pentafluorophenyl)boron. J. Organomet. Chem. 1964, 2, 245–250. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: VII. further studies on tris(pentafluorophenyl)boron. J. Organomet. Chem. 1966, 5, 218–225. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cation-like homogeneous olefin polymerization catalysts based upon zirconocene alkyls and tris(pentafluorophenyl)borane. J. Am. Chem. Soc. 1991, 113, 3623–3625. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Zirconocene Olefin Polymerization Catalysts Based on the Organo-Lewis Acid Tris(pentafluorophenyl)borane. A Synthetic, Structural, Solution Dynamic, and Polymerization Catalytic Study. J. Am. Chem. Soc. 1994, 116, 10015–10031. [Google Scholar] [CrossRef]
- Ewen, J.A.; Elder, M.J. Metallocene Catalysts with Lewis Acids and Aluminum Alkyls. Patent EP0427697, 8 May 1996. [Google Scholar]
- Ewen, J.A.; Elder, M.J. Metallocene Catalysts with Lewis Acids and Aluminum Alkyls. U.S. Patent 5561092, 1 October 1996. [Google Scholar]
- Chien, J.C.W.; Tsai, W.M.; Rausch, M.D. Isospecific polymerization of propylene catalyzed by rac-ethylenebis(indenyl)methylzirconium cation. J. Am. Chem. Soc. 1991, 113, 8570–8571. [Google Scholar] [CrossRef]
- Ewen, J.A.; Elder, M.J. Preparation of metallocene catalysts for polymerization of olefins. Patent EP0426637, 5 April 1995. [Google Scholar]
- Turner, H.W. Catalysts, method of preparing these catalysts and method of using said catalysts. Patent EP0277004, 3 August 1988. [Google Scholar]
- Hlatky, G.; Upton, D.; Turner, H. Supported Ionic Metallocene Catalysts for Olefin Polymerization. Patent WO9109882, 11 July 1991. [Google Scholar]
- Yang, X.; Stern, C.; Marks, T.J. Models for organometallic molecule-support complexes. Very large counterion modulation of cationic actinide alkyl reactivity. Organometallics 1991, 10, 840–842. [Google Scholar] [CrossRef]
- Macchioni, A. Ion Pairing in Transition-Metal Organometallic Chemistry. Chem. Rev. 2005, 105, 2039–2074. [Google Scholar] [CrossRef] [PubMed]
- Ciancaleoni, G.; Fraldi, N.; Budzelaar, P.H.M.; Busico, V.; Cipullo, R.; Macchioni, A. Structure—Activity Relationship in Olefin Polymerization Catalysis: Is Entropy the Key? J. Am. Chem. Soc. 2010, 132, 13651–13653. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Sita, L.R. Ex Uno Plures (“Out of One, Many”): New Paradigms for Expanding the Range of Polyolefins through Reversible Group Transfers. Angew. Chem. Int. Ed. 2009, 48, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Kempe, R. How to Polymerize Ethylene in a Highly Controlled Fashion? Chem. Eur. J. 2007, 13, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- D’Agosto, F.; Boisson, C. A RAFT Analogue Olefin Polymerization Technique Using Coordination Chemistry. Aust. J. Chem. 2010, 63, 1155–1158. [Google Scholar] [CrossRef]
- Drent, E. Process for the Preparation of Polyketones. Patent EP0121965, 17 October 1984. [Google Scholar]
- Drent, E.; Van Broekhoven, J.A.M.; Doyle, M.J. Efficient palladium catalysts for the copolymerization of carbon monoxide with olefins to produce perfectly alternating polyketones. J. Organomet. Chem. 1991, 417, 235–251. [Google Scholar] [CrossRef]
- Drent, E.; Mul, W.P.; Smaardijk, A.A. Polyketones. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Goodall, B.L. Cycloaliphatic Polymers. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 1959–1962. [Google Scholar]
- Makio, H.; Fujita, T. Development and Application of FI Catalysts for Olefin Polymerization: Unique Catalysis and Distinctive Polymer Formation. Acc. Chem. Res. 2009, 42, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Mitani, M.; Saito, J.; Ishii, S.; Nakayama, Y.; Makio, H.; Matsukawa, N.; Matsui, S.; Mohri, J.; Furuyama, R.; Terao, H. FI Catalysts: New olefin polymerization catalysts for the creation of value-added polymers. Chem. Rec. 2004, 4, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Thuilliez, J.; Ricard, L.; Nief, F.; Boisson, F.; Boisson, C. Ansa-Bis(fluorenyl)neodymium Catalysts for Cyclocopolymerization of Ethylene with Butadiene. Macromolecules 2009, 42, 3774–3779. [Google Scholar] [CrossRef]
- Llauro, M.F.; Monnet, C.; Barbotin, F.; Monteil, V.; Spitz, R.; Boisson, C. Investigation of Ethylene/Butadiene Copolymers Microstructure by 1H and 13C NMR. Macromolecules 2001, 34, 6304–6311. [Google Scholar] [CrossRef]
- Belaid, I.; Monteil, V.; Boisson, C. Handbook of Transition Metal Polymerization Catalysts; Hoff, R., Tthers, R., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2017; Chapter 20. [Google Scholar]
- Ribeiro, R.; Ruivo, R.; Nsiri, H.; Norsic, S.; D’Agosto, F.; Perrin, L.; Boisson, C. Deciphering the Mechanism of Coordinative Chain Transfer Polymerization of Ethylene Using Neodymocene Catalysts and Dialkylmagnesium. ACS Catal. 2016, 6, 851–860. [Google Scholar] [CrossRef]
- Zaccaria, F.; Ehm, C.; Budzelaar, P.H.M.; Busico, V. Accurate Prediction of Copolymerization Statistics in Molecular Olefin Polymerization Catalysis: The Role of Entropic, Electronic, and Steric Effects in Catalyst Comonomer Affinity. ACS Catal. 2017, 7, 1512–1519. [Google Scholar] [CrossRef]
- Friederichs, N.; Ghalit, N.; Xu, W. Chptr8—Supported Multicomponent Single-Site α-Olefin Polymerization Catalysts. In Tailor-Made Polymers; Severn, J.R., Chadwick, J.C., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 211–237. [Google Scholar]
- Liu, H.-T.; Davey, C.R.; Shirodkar, P.P. Bimodal polyethylene products from UNIPOL™ single gas phase reactor using engineered catalysts. Macromol. Symp. 2003, 195, 309–316. [Google Scholar] [CrossRef]
- Ruff, M.; Paulik, C. Controlling Polyolefin Properties by In-Reactor Blending, 1–Polymerization Process, Precise Kinetics, and Molecular Properties of UHMW-PE Polymers. Macromol. React. Eng. 2012, 6, 302–317. [Google Scholar] [CrossRef]
- Ruff, M.; Paulik, C. Controlling Polyolefin Properties by In-Reactor Blending: 2. Particle Design. Macromol. React. Eng. 2013, 7, 71–83. [Google Scholar] [CrossRef]
- Ruff, M.; Lang, R.W.; Paulik, C. Controlling Polyolefin Properties by In-Reactor Blending: 3. Mechanical Properties. Macromol. React. Eng. 2013, 7, 328–343. [Google Scholar] [CrossRef]
- Mei, G.; Herben, P.; Cagnani, C.; Mazzucco, A. The Spherizone Process: A New PP Manufacturing Platform. Macromol. Symp. 2006, 245–246, 677–680. [Google Scholar] [CrossRef]
- Dorini, M.; Mei, G. Basell Spherizone Technology. In Sustainable Industrial Chemistry; Cavani, F., Centi, G., Perathoner, S., Trifiró, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 563–578. [Google Scholar]
- Ewen, J.A.; Welborn, J. Process and Catalyst for Producing Polyethylene Having a Broad Molecular Weight Distribution. U.S. Patent 4530914, 23 July 1985. [Google Scholar]
- Ewen, J.A.; Welborn, J. Process and Catalyst for Producing Reactor Blend Polyolefins. U.S. Patent 4937299, 26 June 1990. [Google Scholar]
- Lue, C.-T.; Crowther, D.J. Mixed Catalysts for Use in a Polymerization Process. U.S. Patent 6492472, 10 December 2002. [Google Scholar]
- Vaughan, G.A.; Szul, J.F.; Mckee, M.G.; Farley, J.M.; Lue, C.-T.; Kao, S.-C. Mixed Metallocene Catalyst Systems Containing a Poor Comonomer Incorporator and a Good Comonomer Incorporator. U.S. Patent 7141632, 28 November 2006. [Google Scholar]
- Liu, H.-T.; Mure, C.R. Polyethylene Compositions. U.S. Patent 8378029, 19 February 2013. [Google Scholar]
- Stürzel, M.; Hees, T.; Enders, M.; Thomann, Y.; Blattmann, H.; Mülhaupt, R. Nanostructured Polyethylene Reactor Blends with Tailored Trimodal Molar Mass Distributions as Melt-Processable All-Polymer Composites. Macromolecules 2016, 49, 8048–8060. [Google Scholar] [CrossRef]
- Denger, C.; Haase, U.; Fink, G. Simultaneous oligomerization and polymerization of ethylene. Makromol. Chem. Rapid Commun. 1991, 12, 697–701. [Google Scholar] [CrossRef]
- Ye, Z.; AlObaidi, F.; Zhu, S. A Tandem Catalytic System for the Synthesis of Ethylene–Hex-1-ene Copolymers from Ethylene Stock. Macromol. Rapid Commun. 2004, 25, 647–652. [Google Scholar] [CrossRef]
- Bianchini, C.; Frediani, M.; Giambastiani, G.; Kaminsky, W.; Meli, A.; Passaglia, E. Amorphous Polyethylene by Tandem Action of Cobalt and Titanium Single-Site Catalysts. Macromol. Rapid Commun. 2005, 26, 1218–1223. [Google Scholar] [CrossRef]
- Komon, Z.J.A.; Bu, X.; Bazan, G.C. Synthesis of Butene−Ethylene and Hexene−Butene−Ethylene Copolymers from Ethylene via Tandem Action of Well-Defined Homogeneous Catalysts. J. Am. Chem. Soc. 2000, 122, 1830–1831. [Google Scholar] [CrossRef]
- Komon, Z.J.A.; Diamond, G.M.; Leclerc, M.K.; Murphy, V.; Okazaki, M.; Bazan, G.C. Triple Tandem Catalyst Mixtures for the Synthesis of Polyethylenes with Varying Structures. J. Am. Chem. Soc. 2002, 124, 15280–15285. [Google Scholar] [CrossRef] [PubMed]
- Karbach, F.F.; Macko, T.; Duchateau, R. Preparation of Ethylene/1-Hexene Copolymers from Ethylene Using a Fully Silica-Supported Tandem Catalyst System. Macromolecules 2016, 49, 1229–1241. [Google Scholar] [CrossRef]
- Markel, E.J.; Weng, W.; Peacock, A.J.; Dekmezian, A.H. Metallocene-Based Branch−Block Thermoplastic Elastomers. Macromolecules 2000, 33, 8541–8548. [Google Scholar] [CrossRef]
- Liu, S.; Motta, A.; Delferro, M.; Marks, T.J. Synthesis, Characterization, and Heterobimetallic Cooperation in a Titanium–Chromium Catalyst for Highly Branched Polyethylenes. J. Am. Chem. Soc. 2013, 135, 8830–8833. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Motta, A.; Mouat, A.R.; Delferro, M.; Marks, T.J. Very Large Cooperative Effects in Heterobimetallic Titanium-Chromium Catalysts for Ethylene Polymerization/Copolymerization. J. Am. Chem. Soc. 2014, 136, 10460–10469. [Google Scholar] [CrossRef] [PubMed]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic Production of Olefin Block Copolymers via Chain Shuttling Polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Hustad, P.D.; Kuhlman, R.L.; Arriola, D.J.; Carnahan, E.M.; Wenzel, T.T. Continuous Production of Ethylene-Based Diblock Copolymers Using Coordinative Chain Transfer Polymerization. Macromolecules 2007, 40, 7061–7064. [Google Scholar] [CrossRef]
- Hustad, P.D. Frontiers in Olefin Polymerization: Reinventing the World’s Most Common Synthetic Polymers. Science 2009, 325, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Hustad, P.D.; Marchand, G.R.; Garcia-Meitin, E.I.; Roberts, P.L.; Weinhold, J.D. Photonic Polyethylene from Self-Assembled Mesophases of Polydisperse Olefin Block Copolymers. Macromolecules 2009, 42, 3788–3794. [Google Scholar] [CrossRef]
- Arora, A.; Fosfuri, A.; Gambardella, A. Markets for Technology: The Economics of Innovation and Corporate Strategy; MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Chaudhary, B.I.; Barry, R.P. Extruded Non-Crosslinked Foams Made from Ethylene-Styrene Interpolymers and Blends with Polyethylene. J. Cell. Plast. 1999, 35, 531–549. [Google Scholar]
- Timmers, F.J. Pseudo-Random Copolymers Formed by Use of Constrained Geometry Addition Polymerization Catalysts. U.S. Patent 5703187, 30 December 1997. [Google Scholar]
- Chum, P.S.; Kruper, W.J.; Guest, M.J. Materials Properties Derived from INSITE Metallocene Catalysts. Adv. Mater. 2000, 12, 1759–1767. [Google Scholar] [CrossRef]
- Thayer, A.M. Metallocene Catalysts Initiate New Era in Polymer Synthesis. Chem. Eng. News Arch. 1995, 73, 15–20. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but Not Gagged”Immobilizing Single-Site α-Olefin Polymerization Catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef] [PubMed]
- Severn, J.R.; Chadwick, J.C. Immobilisation of homogeneous olefin polymerisation catalysts. Factors influencing activity and stability. Dalton Trans. 2013, 42, 8979–8987. [Google Scholar] [CrossRef] [PubMed]
- Busico, V. Catalytic Olefin Polymerization is a Mature Field. Isn’t it? Macromol. Chem. Phys. 2007, 208, 26–29. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060185
Sauter DW, Taoufik M, Boisson C. Polyolefins, a Success Story. Polymers. 2017; 9(6):185. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060185
Chicago/Turabian StyleSauter, Dominique W., Mostafa Taoufik, and Christophe Boisson. 2017. "Polyolefins, a Success Story" Polymers 9, no. 6: 185. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9060185