Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrogel and Bio-Signal Electrode
- (1)
- Raw polymers of PVA-1799 (4.00 g, –[CH2–CH(OH)]n–, average value of molecular weight is 1799) and PEG-6000 (4.00 g, HO–(CH2–CH2–O)n–H, average value of molecular weight is 6000) were blended into a flask with three necks, then 20.00 mL of deionized water was dropped into the flask;
- (2)
- The solution was stirred at a rate of 600 r/min for 10 min at 90 °C, a transparent mixture of PVA-1799/PEG-6000 solution was then obtained;
- (3)
- Stirring was continue at the rate of 600 r/min without heating; on the other hand, 0.30 g GO was dispersed into 10.00 mL deionized water under ultrasonic dispersion for 60 min;
- (4)
- The dispersed GO solution was put into the stirring PVA-1799/PEG-6000 mixed solution, maintaining the stirring at a rate of 600 r/min for 30 min;
- (5)
- The blended solution was poured into a PVDF pipe with an inner diameter of 10.00 mm for sample #1. The solution was then customed into an organic glass mold where a nonwoven fabric was in the mold as a substrate for sample #2. Then the pipe and mold were sealed with preservative films;
- (6)
- The PVDF pipe and the organic glass mold were placed into a freezer which could supply a cold environment of −40 °C. The samples were maintained in such an environment for 8 h, then removed from the freezer for thawing for 4 h at room temperature;
- (7)
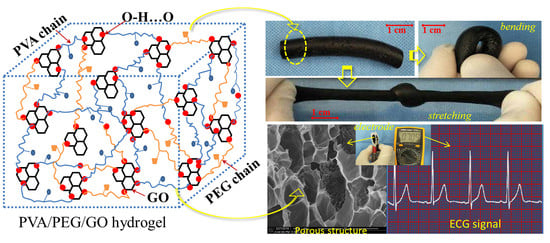
- The freezing–thawing process was repeated three times for the generation of the PVA/PEG/GO nanocomposite hydrogel. An illustration for such a cyclic freezing-thawing method in preparing PVA/PEG/GO hydrogel is shown in Figure 1, where the raw materials of PEG and PVA were blended in hot water. After the addition of GO nanoparticles, the freezing and thawing processes were performed until the last image, which shows a large amount of crosslinking generated for high mechanical performance. Here, for the sake of comparison, the solution of step (2) can also experience the steps (5–7) for preparing PVA/PEG hydrogel. The hydrogel is then compared with the PVA/PEG/GO hydrogel in terms of mechanical properties and electrical conductivity.
2.3. Hydrogel Characterization
3. Results and Discussions
3.1. Synthesis and Morphology Analysis of the PVA/PEG/GO Hydrogel
3.2. Characterization of Crosslinking Formation of the PVA/PEG/GO Hydrogel

3.3. Analysis of Mechanical Properties
3.4. Electrical Conductivity of PVA/PEG/GO Hydrogel and Its Application in ECG Acquisition
3.4.1. Conductivity of PVA/PEG/GO Hydrogels
3.4.2. Hydrogel in Human ECG Signal Acquisition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munro, H.S.; Yasin, M. Bioadhesive Composition and Biomedical Electrodes Containing Them. U.S. Patent 7076282, 17 July 2006. [Google Scholar]
- Kosierkiewicz, T.A. Dry and flexible elastomer electrodes outperform similar hydrogel and Ag/AgCl electrodes. In Proceedings of the IEEE International Symposium on Medical Measurements & Applications, Gatineau, QC, Canada, 4–5 May 2013; pp. 306–308. [Google Scholar]
- Crenner, F.; Angel, F.; Ringwald, C. Ag/AgCl electrode assembly for thin smooth muscle electromyography. Med. Biol. Eng. Comput. 1989, 27, 346–356. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Lowery, M.M.; Doherty, L.S.; McHugh, M.; O’Muircheartaigh, C.; Cullen, J.; Nolan, P.; McNicholas, W.T.; O’Malley, M.J. Improved surface EMG electrode for measuring genioglossus muscle activity. Respir. Physiol. Neurobiol. 2007, 159, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Cui, W.B. Analysis and discussion on skin allergy caused by disposable electrocardiogram electrode. Chin. J. Pharmacovigil. 2014, 11, 35–42. [Google Scholar]
- Dou, Q.Q.; Karim, A.; Loh, X.J. Modification of thermal and mechanical properties of PEG–PPG–PEG Copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. [Google Scholar] [CrossRef]
- Pattananuwat, P.; Aht-Ong, D. One-step method to fabricate the highly porous layer of poly (pyrrole/(3,4-ethylenedioxythiophene)) wrapped graphene hydrogel composite electrode for the flexible supercapacitor. Mater. Lett. 2016, 184, 60–64. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zeng, S.; Chen, M.H.; Zhang, Y.Y.; Zheng, L.X.; Li, Q.W. Oxygen Evolution Assisted Fabrication of Highly Loaded Carbon Nanotube/MnO2 Hybrid Films for High-Performance Flexible Pseudosupercapacitors. Small 2016, 12, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.S.; Pei, D.F.; Jiang, W.; Mu, Y.B.; Wan, X.B. A simple and fast formation of biodegradable poly(urethane-urea) hydrogel with high water content and good mechanical property. Polymer 2016, 99, 340–348. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Milani, M.A.; Jelvehgari, M. Hydrogel nanoparticles and nanocomposites for nasal drug/vaccine delivery. Arch. Pharmacal Res. 2016, 39, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Mary, D.S.; Kannan, S. Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int. J. Biol. Macromol. 2017, 95, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Pensalfini, M.; Ehret, A.E.; Studeli, S.; Marino, D.; Kaech, A.; Reichmann, E.; Mazza, E. Factors affecting the mechanical behavior of collagen hydrogels for skin tissue engineering. J. Mech. Behav. Biomed. Mater. 2017, 69, 85–97. [Google Scholar] [CrossRef] [PubMed]
- England, A.H.; Clare, T.L. Synthesis and Characterization of Flexible Hydrogel Electrodes for Electrochemical Impedance Measurements of Protective Coatings on Metal Sculptures. Electroanalysis 2014, 26, 1059–1067. [Google Scholar] [CrossRef]
- Azmi, S.; Abd Razak, S.I.; Kadir, M.R.A.; Iqbal, N.; Hassan, R.; Nayan, N.H.M.; Wahab, A.H.A.; Shaharuddin, S. Reinforcement of poly(vinyl alcohol) hydrogel with halloysite nanotubes as potential biomedical materials. Soft Mater. 2017, 15, 45–54. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Huang, H.B.; Yao, J.L.; Li, L.; Zhu, F.; Liu, Z.T.; Zeng, X.P.; Yu, X.H.; Huang, Z.L. Reinforced polyaniline/polyvinyl alcohol conducting hydrogel from a freezing-thawing method as self-supported electrode for supercapacitors. J. Mater. Sci. 2016, 51, 8728–8736. [Google Scholar] [CrossRef]
- Bijpai, A.K.; Saini, R. Preparation and characterization of novel biocompatible cryogels of poly (vinyl alcohol) and egg-albumin and their water sorption study. J. Mater. Sci. Mater. Med. 2006, 17, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suo, J.P.; Deng, R.Z. Structure, Mechanical, and Swelling Behaviors of Poly(vinyl alcohol)/SiO2 Hybrid Membranes. J. Reinf. Plast. Compos. 2010, 29, 618–629. [Google Scholar]
- Holloway, J.L.; Lowman, A.M.; VanLandingham, M.R.; Palmese, G.R. Chemical grafting for improved interfacial shear strength in UHMWPE/PVA-hydrogel fiber-based composites used as soft fibrous tissue replacements. Compos. Sci. Technol. 2013, 85, 118–125. [Google Scholar] [CrossRef]
- Calles, J.A.; Tartara, L.I.; Lopez-Garcia, A.; Diebold, Y.; Palma, S.D.; Valles, E.M. Novel bioadhesive hyaluronan-itaconic acid crosslinked films for ocular therapy. Int. J. Pharm. 2013, 455, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Owh, C.; Jiang, S.; Ng, C.Z.Q.; Wirawan, D.; Loh, X.J. A Thixotropic Polyglycerol Sebacate-Based Supramolecular Hydrogel as an Injectable Drug Delivery Matrix. Polymers 2016, 8, 130. [Google Scholar] [CrossRef]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Yin, H.M.; Qian, J.; Zhang, J.; Lin, Z.F.; Li, J.S.; Xu, J.Z.; Li, Z.M. Engineering Porous Poly(lactic acid) Scaffolds with High Mechanical Performance via a Solid State Extrusion/Porogen Leaching Approach. Polymers 2016, 8, 125. [Google Scholar] [CrossRef]
- Zhao, F.L.; Yao, D.; Guo, R.W.; Deng, L.D.; Dong, A.J.; Zhang, J.H. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Zou, M.C.; Wu, S.T.; Xu, W.J.; Wu, H.S.; Cao, A.Y. Graphene Oxide Glue-Electrode for Fabrication of Vertical, Elastic, Conductive Columns. ACS Nano 2017, 11, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Rudra, R.; Kumar, V.; Pramanik, N.; Jundu, P.P. Graphite oxide incorporated crosslinked polyvinyl alcohol and sulfonated styrene nanocomposite membrane as separating barrier in single chambered microbial fuel cell. J. Power Sources 2017, 341, 285–293. [Google Scholar] [CrossRef]
- Chen, L.L.; Liu, J.; Fang, X.M.; Zhang, Z.G. Reduced graphene oxide dispersed nanofluids with improved photo thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Kalluru, P.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.S.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Modi, G.; Naidoo, D.; Kumar, P. A Polyvinyl Alcohol-Polyaniline Based Electro-Conductive Hydrogel for Controlled Stimuli-Actuable Release of Indomethacin. Polymers 2011, 3, 150–172. [Google Scholar] [CrossRef]
- Liu, C.P.; Gao, X.P.; Liu, J. Preparation and properties of polyvinyl alcohol/gelatin/graphene oxide nanocomposite hydrogels. J. Polym. Mater. Sci. Eng. 2015, 8, 156–161. [Google Scholar]
- Dai, M.; Xiao, X.; Chen, X.; Lin, H.M.; Wu, W.; Chen, S.P. A low-power and miniaturized electrocardiograph data collection system with smart textile electrodes for monitoring of cardiac function. Australas. Phys. Eng. Sci. Med. 2017, 39, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, J.; Gui, X.; Qian, K. Shape memory investigation of α-keratin fibers as multi-coupled stimuli of responsive smart materials. Polymers 2017, 9, 87. [Google Scholar] [CrossRef]
- Ashrafizadeh, H.; Mertiny, P.; McDonald, A. Evaluation of the effect of temperature on mechanical properties and wear resistance of polyurethane elastomers. Wear 2016, 368, 26–38. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Yokota, T.; Kuribara, K.; Kaltenbrunner, M.; Fukushima, T.; Inoue, Y.; Sekino, M.; Isoyama, T.; Abe, Y.; Onodera, H.; et al. Ultraflexible organic amplifier with biocompatible gel electrodes. Nat. Commun. 2016, 7, 11425. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-flexibility, high-toughness double-cross- linked chitin hydrogels by sequential chemical and physical cross-linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
 means freezing process, and
means freezing process, and  means thawing process).
means thawing process).
 means freezing process, and
means freezing process, and  means thawing process).
means thawing process).







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Wu, G.; Zhou, H.; Qian, K.; Hu, J. Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Polymers 2017, 9, 259. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9070259
Xiao X, Wu G, Zhou H, Qian K, Hu J. Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Polymers. 2017; 9(7):259. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9070259
Chicago/Turabian StyleXiao, Xueliang, Guanzheng Wu, Hongtao Zhou, Kun Qian, and Jinlian Hu. 2017. "Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition" Polymers 9, no. 7: 259. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9070259