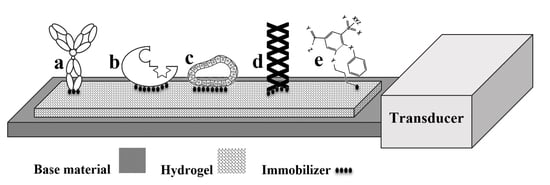
Hydrogel Based Sensors for Biomedical Applications: An Updated Review
Abstract
:1. Introduction
2. Bioreceptors
3. Hydrogels for Biosensing
3.1. Polyvinyl Alcohol
3.2. Polyethylene Glycol
3.3. Polyacrylate Families
3.4. Hydrogels with Biological Origin
3.5. Electroconductive Hydrogels
4. Immobilization Techniques
5. Transducing Strategies
6. Biomedical Applications of Biosensors
6.1. Cell Metabolite Detection
6.2. Tissue Engineering
6.3. Wound Healing Biosensors
6.4. Cancer Monitoring
6.5. Pathogen Detection
6.6. Detection of Small Molecules
7. Future Outlook
Author Contributions
Conflicts of Interest
References
- Vo-Dinh, T. Nanobiosensors: Probing the sanctuary of individual living cells. J. Cell. Biochem. 2002, 87, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Mattiasson, B. Molecular imprinting techniques used for the preparation of biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E.; Tavakoli, J.; Sarvestani, A.S. Swelling characteristics of acrylic acid polyelectrolyte hydrogel in a dc electric field. Smart Mater. Struct. 2007, 16, 1614. [Google Scholar] [CrossRef]
- Cretich, M.; Pirri, G.; Damin, F.; Solinas, I.; Chiari, M. A new polymeric coating for protein microarrays. Anal. Biochem. 2004, 332, 67. [Google Scholar] [CrossRef] [PubMed]
- Dehbari, N.; Tavakoli, J.; Zhao, J.; Tang, Y. In situ formed internal water channels improving water swelling and mechanical properties of water swellable rubber composites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in vitro biomedical studies. Mater. Sci. Eng 2017, 77, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Duan, J.; Tang, Y.; Qiao, S.Z. Hybrid hydrogels of porous graphene and nickel hydroxide as advanced supercapacitor materials. Chem. Eur. J. 2013, 19, 7118–7124. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Yu, Y.; Chen, S.; Yang, Y.; Tang, Y. Conductive nanocomposite hydrogels with self-healing property. RSC Adv. 2014, 4, 35149–35155. [Google Scholar] [CrossRef]
- Salina, M.; Giavazzi, F.; Lanfranco, R.; Ceccarello, E.; Sola, L.; Chiari, M.; Chini, B.; Cerbino, R.; Bellini, T.; Buscaglia, M. Multi-spot, label-free immunoassay on reflectionless glass. Biosens. Bioelectron. 2015, 74, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nicu, L.; Leïchlé, T. Biosensors and tools for surface functionalization from the macro-to the nanoscale: The way forward. J. Appl. Phys. 2008, 104, 12. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Caygill, R.L.; Blair, G.E.; Millner, P.A. A review on viral biosensors to detect human pathogens. Anal. Chim. Acta 2010, 681, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody production, design and use for biosensor-based applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Pita, M.; Minko, S.; Katz, E. Enzyme-based logic systems and their applications for novel multi-signal-responsive materials. J. Mater. Sci. 2008, 20, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Gaspar, S.; Leth, S.; Niculescu, M.; Mortari, A.; Bontidean, I.; Soukharev, V.; Someanu, S.A.; Ryabov, A.D.; Csoregi, E. Biosensors for life quality: Design, development and applications. Sens. Actuator B 2004, 102, 179–194. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Review: Recent developments in enzyme-based biosensors for biomedical analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Sassolas, A.; Leca-Bouvier, B.D.; Blum, L.J. DNA biosensors and microarrays. Chem. Rev. 2008, 108, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, K.J.; Gooding, J.J. An introduction to electrochemical DNA biosensors. Analyst 2007, 132, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M.; Hartig, J.S.; Mayer, G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007, 107, 3715–3743. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Pino, L.; Strambini, L.M.; Tedeschi, L.; Barillaro, G. 10000-fold improvement in protein detection using nanostructured porous silicon interferometric aptasensors. ACS Sensors 2016, 1, 1471–1479. [Google Scholar] [CrossRef]
- Wang, P.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and its application in biomedicine. Sens. Actuator B 2005, 108, 576–584. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.J.; Kanakasabapathy, M.K.; Verma, S.; Chug, M.K.; Memic, A.; Gadjeva, M.; Shafiee, H. Label-free electrical sensing of bacteria in eye wash samples: A step towards point-of-care detection of pathogens in patients with infectious keratitis. Biosens. Bioelectron. 2017, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev. Technol. 2006, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Lenz, D.; Robinson, J.P.; Rickus, J.L.; Bhunia, A.K. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab. Investig. 2008, 88, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Bhunia, A.K. Mammalian cell-based biosensors for pathogens and toxins. Trends Biotechnol. 2009, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Baldini, L.; Casnati, A.; Ungaro, R. Calixarenes: From biomimetic receptors to multivalent ligands for biomolecular recognition. New J. Chem. 2010, 34, 2715–2728. [Google Scholar] [CrossRef]
- Kowalczyk, S.W.; Blosser, T.R.; Dekker, C. Biomimetic nanopores: Learning from and about nature. Trends Biotechnol. 2011, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mecke, A.; Dittrich, C.; Meier, W. Biomimetic membranes designed from amphiphilic block copolymers. Soft Matt. 2006, 2, 751–759. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, C.; Chopineau, J. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur. Biophys. J. 2007, 36, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Wackerlig, J.; Lieberzeit, P.A. Biomimetic strategies for sensing biological species. Biosensors 2013, 3, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Andrade, C.A.; Oliveira, M.D.; Sun, X.L. Carbohydrate–protein interactions and their biosensing applications. Anal. Bioanal. Chem. 2012, 402, 3161–3176. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Audfray, A.; Imberty, A. Binding sugars: From natural lectins to synthetic receptors and engineered neolectins. Chem. Soc. Rev. 2013, 42, 4798–4813. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.Y.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef] [PubMed]
- El-Sharif, H.; Stevenson, D.; Reddy, S. MIP-based protein profiling: A method for interspecies discrimination. Sens. Actuators B 2017, 241, 33–39. [Google Scholar] [CrossRef]
- Song, H.S.; Kwon, Q.S.; Kim, J.H.; Conde, J.; Artzi, N. 3D hydrogel scaffold doped with 2D graphene materials for biosensors and bioelectronics. Biosens. Bioelectron. 2017, 89, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Skottrup, P.D.; Nicolaisen, M.; Justesen, A.F. Towards on-site pathogen detection using antibody-based sensors. Biosens. Bioelectron. 2008, 24, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Kotanen, C.N.; Moussy, F.G.; Carrara, S.; Guiseppi-Elie, A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 2012, 35, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Kieninger, J.; Urban, G.A. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal. Bioanal. Chem. 2016, 408, 4503–4521. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors. Soft Mater. 2011, 7, 6757–6767. [Google Scholar] [CrossRef]
- Sefah, K.; Philips, J.A.; Xiong, X.; Meng, L.; Van Simaeys, D.; Chen, H.; Martin, J.; Tan, W. Nucleic acid aptamers for biosensors and bio-analytical applications. Analyst 2009, 134, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, X.; Xie, C.; Wan, J.; Zhang, H.; Tang, Y. Flexible, free-standing TiO2–graphene–polypyrrole composite films as electrodes for supercapacitors. J. Phys. Chem. C 2015, 119, 3903–3910. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic mineralized hierarchical graphene oxide/chitosan scaffolds with adsorbability for immobilization of nanoparticles for biomedical applications. ACS Appl. Mater. Interface 2016, 8, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, A.; Damin, F.; Özkumur, E.; di Carlo, G.; Goldberg, B.B.; Chiari, M.; Ünlü, M.S. Direct observation of conformation of a polymeric coating with implications in microarray applications. Anal. Chem. 2009, 81, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ryu, M.; Rark, C.Y.; Ahn, J.; Park, H.G.; Choi, C.; Ha, S.D.; Park, T.J.; Park, J.P. High sensitive and selective electrochemical biosensor: Label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017, 87, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Jabbari, E.; Khosroshahi, M.E.; Boroujerdi, M. Swelling characterization of anionic acrylic acid hydrogel in an external electric field. Iran. Polym. J. 2006, 15, 891–897. [Google Scholar]
- Liu, L.; Li, L.; Qing, Y.; Yan, N.; Wu, Y.; Li, X.; Tian, C. Mechanically strong and thermosensitive hydrogels reinforced with cellulose nanofibrils. Polym. Chem. 2016, 7, 7142–7151. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Singh, H.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Enhanced glucose sensor linearity using poly(vinyl alcohol) hydrogels. J. Diabetes Sci. Technol. 2009, 3, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Su, X.; Wei, J.; Ren, X.; Li, L.; Meng, X.; Ren, J.; Tang, F. A new amperometric glucose biosensor based on one-step electrospun poly(vinyl alcohol)/chitosan nanofibers. J. Biomed. Nanotechnol. 2013, 9, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Guascito, M.R.; Chirizzi, D.; Malitesta, C.; Mazzotta, E. Mediator-free amperometric glucose biosensor based on glucose oxidase entrapped in poly(vinyl alcohol) matrix. Analyst 2011, 136, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, Z.; Wang, Q.; Zheng, J. A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles–carbon nanotubes composite film. Sens. Actuators B 2011, 158, 23–27. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhou, T.Y.; Chen, X.; Wong, K.Y.; Wang, X.R. An optical biosensor for the rapid determination of glucose in human serum. Sens. Actuators B 2008, 129, 866–873. [Google Scholar] [CrossRef]
- Wu, J.; Yin, F. Sensitive enzymatic glucose biosensor fabricated by electrospinning composite nanofibers and electrodepositing Prussian blue film. J. Electroanal. Chem. 2013, 694, 1–5. [Google Scholar] [CrossRef]
- Pundir, C.S.; Singh, B.S.; Narang, J. Construction of an amperometric triglyceride biosensor using PVA membrane bound enzymes. Clin. Biochem. 2010, 43, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Bertok, T.; Gemeiner, P.; Mikula, M.; Gemeiner, P.; Tkac, J. Ultrasensitive impedimetric lectin based biosensor for glycoproteins containing sialic acid. Microchim. Acta 2013, 180, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Huang, J.D.; Chiu, C.C. Amperometric ethanol biosensor based on poly(vinyl alcohol)-multiwalled carbon nanotube-alcohol dehydrogenase biocomposite. Biosens. Bioelectron. 2007, 22, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Topkar, A.; D’Souza, S.F. Development of potentiometric urea biosensor based on urease immobilized in PVA–PAA composite matrix for estimation of blood urea nitrogen (BUN). J. Biochem. Biophys. Methods 2008, 70, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Dehdast, A.; Zarei, H.; Sourkohi, B.K.; Larijani, B. Development of urinary albumin immunosensor based on colloidal AuNP and PVA. Biosens. Bioelectron. 2011, 26, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Doong, R. Preparation and characterization of urease-encapsulated biosensors in poly(vinyl alcohol)-modified silica sol–gel materials. Biosens. Bioelectron. 2007, 23, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. Portable urea biosensor based on the extended-gate field effect transistor. Sens. Actuators B 2003, 91, 180–186. [Google Scholar] [CrossRef]
- Tan, S.; Tan, X.; Xu, J.; Zhao, D.; Zhang, J.; Liu, L. A novel hydrogen peroxide biosensor based on sol-gel poly (vinyl alcohol) (PVA)/(titanium dioxide)TiO2 hybrid material. Anal. Methods 2011, 3, 110–115. [Google Scholar] [CrossRef]
- Fan, C.; Gao, Q.; Zhu, D.; Wagner, G.; Li, G. An unmediated hydrogen peroxide biosensor based on hemoglobin incorporated in a montmorillonite membrane. Analyst 2001, 126, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, A.M.; Mardlin, D.P.; Turner, N.L.; Henkler, R.; Strachan, N.; Glover, L.A.; Paton, G.I.; Killham, K. On-line microbial biosensing and fingerprinting of water pollutants. Biosens. Bioelectron. 2002, 17, 495–501. [Google Scholar] [CrossRef]
- Rainina, E.I.; Badalian, I.E.; Ignatov, O.V.; Fedorov, A.Y.; Simonian, A.L.; Varfolomeyev, S.D. Cell biosensor for detection of phenol in aqueous solutions. Appl. Biochem. Biotechnol. 1996, 56, 117–127. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Chen, C.; Shen, J.; Bao, H.; Peng, L.; Yang, X.; Li, C. Photonic crystal pH and metal cation sensors based on poly(vinyl alcohol) hydrogel. New J. Chem. 2012, 36, 1051–1056. [Google Scholar] [CrossRef]
- Pang, J.; Fan, C.; Liu, X.; Chen, T.; Li, G. A nitric oxide biosensor based on the multi-assembly of hemoglobin/montmorillonite/polyvinyl alcohol at a pyrolytic graphite electrode. Biosens. Bioelectron. 2003, 19, 441–445. [Google Scholar] [CrossRef]
- Muñoz, E.M.; Yu, H.; Hallock, J.; Edens, R.E.; Linhardt, R.J. Poly(ethylene glycol)-based biosensor chip to study heparin–protein interactions. Anal. Biochem. 2005, 343, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Riedelova-Reicheltova, Z.; Majek, P.; Eodriguez-Emmenegger, C.; Houska, M.; Dyr, J.E.; Brynda, E. Complete identification of proteins responsible for human blood plasma fouling on poly(ethylene glycol)-based surfaces. Langmuir 2013, 29, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Sun, C.; Miao, J.; Yan, J.; Yang, K.; Mao, C.; Ju, J.; Shen, J. Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood. Electrochim. Acta 2013, 89, 549–554. [Google Scholar] [CrossRef]
- Yan, J.; Pedrosa, V.A.; Enomoto, J.; Simonian, A.L.; Revzin, A. Electrochemical biosensors for on-chip detection of oxidative stress from immune cells. Biomicrofluidics 2011, 5, 032008. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.M.; Abdullah, J.; Yusof, N.A.; Ab Rashid, A.H.; Abd Rahman, S.; Hasan, M.R. Amperometric biosensor based on zirconium oxide/polyethylene glycol/tyrosinase composite film for the detection of phenolic compounds. Biosensors 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.P.O.; Meledeo, M.A.; Davis, J.R.; Ibey, B.L.; Gant, V.A.; Pishko, M.V.; Cote, G.L. Oxygen sensor based on the fluorescence quenching of a ruthenium complex immobilized in a biocompatible poly(ethylene glycol) hydrogel. IEEE Sens. J. 2004, 4, 728–734. [Google Scholar] [CrossRef]
- Dey, P.; Adamovski, M.; Friebe, S.; Badalyan, A.; Mutihac, R.C.; Paulus, F.; Leimkuhler, S.; Wollenberger, U.; Haag, R. Dendritic polyglycerol–poly(ethylene glycol)-based polymer networks for biosensing application. ACS Appl. Mater. Interfaces 2014, 6, 8937–8941. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Song, Z.; Wu, Y.; Guo, B.; Fan, X.; Luo, X. A highly sensitive biosensor for tumor maker alpha fetoprotein based on poly(ethylene glycol) doped conducting polymer PEDOT. Biosens. Bioelectron. 2016, 79, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, H.; Bozokalfa, G.; Asker, D.N.; Demir, B.; Guler, E.; Odaci Demirkol, D.; Timur, S.; Yagci, Y. Polythiophene-g-poly(ethylene glycol) with lateral amino groups as a novel matrix for biosensor construction. ACS Appl. Mater. Interfaces 2015, 7, 20612–20622. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, M.; Seo, Y.T.; Yun, K.; Lee, M.H. Graphene oxide functionalized with silver@silica–polyethylene glycol hybrid nanoparticles for direct electrochemical detection of quercetin. Biosens. Bioelectron. 2014, 58, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Zaitouna, A.J.; Joyce, J.; Cerny, R.L.; Dussault, P.H.; Lai, R.Y. Comparison of mannose, ethylene glycol, and methoxy-terminated diluents on specificity and selectivity of electrochemical peptide-based sensors. Anal. Chem. 2015, 87, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová, H.; Brynda, E.; Homola, J. Functionalizable low-fouling coatings for label-free biosensing in complex biological media: Advances and applications. Anal. Bioanal. Chem. 2015, 407, 3927–3953. [Google Scholar] [CrossRef] [PubMed]
- Elhag, S.; Khun, K.; Khranovskyy, V.; Liu, X.; Willander, M.; Nur, O. Efficient donor impurities in ZnO nanorods by polyethylene glycol for enhanced optical and glutamate sensing properties. Sensors 2016, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Homayoun, A.; Bannister, C.W.; Yum, K. Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine. Biotechnol. J. 2015, 10, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Anderson, M.; Bernards, M.T.; Hunt, H.K. PEG functionalization of whispering gallery mode optical microresonator biosensors to minimize non-specific adsorption during targeted, label-free sensing. Sensors 2015, 15, 18040–18060. [Google Scholar] [CrossRef] [PubMed]
- Orgovan, N.; Peter, B.; Bosze, S.; Ramsden, J.J.; Szabo, B.; Horvath, R. Dependence of cancer cell adhesion kinetics on integrin ligand surface density measured by a high-throughput label-free resonant waveguide grating biosensor. Sci. Rep. 2014, 4, 4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Halhouli, A.; Demming, S.; Alahmad, L.; Llpbera, A.; Buttgenbach, S. An in-line photonic biosensor for monitoring of glucose concentrations. Sensors 2014, 14, 15749–15759. [Google Scholar] [CrossRef] [PubMed]
- Kermis, H.R.; Kostov, Y.; Hams, P.; Rao, G. Dual excitation ratiometric fluorescent pH sensor for noninvasive bioprocess monitoring: Development and application. Biotechnol. Prog. 2002, 18, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, V.K.; Koh, W.G.; Lazur, G.J.; Pishko, M.V. Microfabricated protein-containing poly(ethylene glycol) hydrogel arrays for biosensing. Sens. Actuators B 2004, 97, 290–297. [Google Scholar] [CrossRef]
- Su, L.C.; Tian, Y.C.; Chang, Y.E.; Chou, C.; Lai, C.S. Rapid detection of urinary polyomavirus BK by heterodyne-based surface plasmon resonance biosensor. J. Biomed. Opt. 2013, 19, 011013. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qi, L.; Shen, Y.; Qiao, J.; Chen, Y. Novel oligo(ethylene glycol)-based molecularly imprinted magnetic nanoparticles for thermally modulated capture and release of lysozyme. ACS Appl. Mater. Interfaces 2014, 6, 17289–17295. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, D.; Zhang, Q.; Lu, Y.; Li, N.; Chen, Q.; Liu, Q. Electrophoresis-enhanced localized surface plasmon resonance sensing based on nanocup array for thrombin detection. Sens. Actuators B 2016, 232, 219–225. [Google Scholar] [CrossRef]
- Lee, S.; Ibey, B.L.; Cote, G.L.; Pishko, M.V. Measurement of pH and dissolved oxygen within cell culture media using a hydrogel microarray sensor. Sens. Actuators B 2008, 128, 388–398. [Google Scholar] [CrossRef]
- Yin, M.-J.; Yao, M.; Gao, S.; Zhang, A.P.; Tam, H.Y.; Wai, P.K.A. Rapid 3D patterning of poly(acrylic acid) ionic hydrogel for miniature pH sensors. Adv. Mater. 2016, 28, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Dey, J.; Adhikari, B. Taste sensing with polyacrylic acid grafted cellulose membrane. Talanta 2006, 69, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Kikuchi, M.; Yamazaki, M.; Shiratori, S. Ammonia sensors based on electrospun poly(acrylic acid) fibrous membranes. IEEE Sens. 2004, 682, 685–688. [Google Scholar]
- Nickel, A.-M.L.; Seker, F.; Ziemer, B.P.; Ellis, A.B. Imprinted poly(acrylic acid) films on cadmium selenide; A composite sensor structure that couples selective amine binding with semiconductor substrate photoluminescence. Chem. Mater. 2001, 13, 1391–1397. [Google Scholar] [CrossRef]
- Suri, J.T.; Cordes, D.B.; Cappuccio, F.E.; Wessling, R.A.; Singaram, B. Continuous glucose sensing with a fluorescent thin-film hydrogel. Angew. Chem. Int. Ed. 2003, 42, 5857–5859. [Google Scholar] [CrossRef] [PubMed]
- Vilozny, B.; Schiller, A.; Wessling, R.A.; Singaram, B. Multiwell plates loaded with fluorescent hydrogel sensors for measuring pH and glucose concentration. J. Mater. Chem. 2011, 21, 7589–7595. [Google Scholar] [CrossRef]
- Yang, X.; Pan, X.; Blyth, J.; Lowe, C.R. Towards the real-time monitoring of glucose in tear fluid: Holographic glucose sensors with reduced interference from lactate and pH. Biosens. Bioelectron. 2008, 23, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Wencel, D.; Abel, T.; McDonagh, C. Optical chemical pH sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dong, X.; Wong, W.C.; Chen, L.H.; Ni, K.; Chan, C.C. Photonic crystal fiber interferometric pH sensor based on polyvinyl alcohol/polyacrylic acid hydrogel coating. Appl. Opt. 2015, 54, 2647–2652. [Google Scholar] [CrossRef] [PubMed]
- Sorber, J.; Steiner, G.; Schulz, V.; Guenther, M.; Gerlach, G.; Salzer, R.; Arndt, K.-F. Hydrogel-based piezoresistive pH sensors: Investigations using FT-IR attenuated total reflection spectroscopic imaging. Anal. Chem. 2008, 80, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Hilt, J.Z.; Steiner, G.; Schulz, V.; Guenther, M.; Garlach, G.; Salzer, R.; Arndt, K.E. Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels. Biomed. Microdevices 2003, 5, 177–184. [Google Scholar] [CrossRef]
- McCoy, C.P.; Stomeo, F.; Plush, S.; Gunnlaugsson, T. Soft matter pH sensing: From luminescent lanthanide pH switches in solution to sensing in hydrogels. Chem. Mater. 2006, 18, 4336–4343. [Google Scholar] [CrossRef]
- Korostynska, O.; Arshak, K.; Gill, E.; Arshak, A. Review on state-of-the-art in polymer based pH sensors. Sensors 2007, 7, 3027–3042. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.F.; Snow, D.; Sterling, R.; Brown, G.M. A pH sensor based on a microcantilever coated with intelligent hydrogel. Instrum. Sci. Technol. 2004, 32, 361–369. [Google Scholar] [CrossRef]
- Herber, S.; Bomer, J.; Olthuis, W.; Bergveld, P.; Berg, A.V.D. A miniaturized carbon dioxide gas sensor based on sensing of pH-sensitive hydrogel swelling with a pressure sensor. Biomed. Microdevices 2005, 7, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.J.; Bomer, J.; Olthuis, W.; Bergveld, P.; van de Berg, A. pH-sensitive holographic sensors. Anal. Chem. 2003, 75, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, B.D. Fabrication and characterization of a highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing high index layer and smart hydrogel. Sens. Actuators B 2012, 173, 268–273. [Google Scholar] [CrossRef]
- Ruan, C.; Zeng, K.; Grimes, C.A. A mass-sensitive pH sensor based on a stimuli-responsive polymer. Anal. Chim. Acta 2003, 497, 123–131. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, J.; Fang, K. New technology for the detection of pH. J. Biochem. Biophys. Methods 2005, 63, 1–9. [Google Scholar]
- Shin, J.; Han, S.G.; Lee, W. Inverse opal pH sensors with various protic monomers copolymerized with polyhydroxyethylmethacrylate hydrogel. Anal. Chim. Acta 2012, 752, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Yin, M.J.; Zhang, A.P.; Qian, J.W.; He, S. Low-cost high-performance fiber-optic pH sensor based on thin-core fiber modal interferometer. Opt. Exp. 2009, 17, 22296–22302. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Lamm, M.S.; Haines-Butterich, L.A.; Pochan, D.J.; Schneider, J.P. Tuning the pH responsiveness of β-hairpin peptide folding, self-assembly, and hydrogel material formation. Biomacromolecules 2009, 10, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Aigner, D.; Borisov, S.; Petritsch, P.; Klimant, I. Novel near infra-red fluorescent pH sensors based on 1-aminoperylene bisimides covalently grafted onto poly(acryloylmorpholine). Chem. Commun. 2013, 49, 2139–2141. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, B.; Dong, Y.; Chen, F.; Li, Y.; Li, Z.; He, J.; Li, H.; Tian, W. Novel fluorescent pH sensors and a biological probe based on anthracene derivatives with aggregation-induced emission characteristics. Langmuir 2010, 26, 6838–6844. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Takahata, K. A hydrogel-based passive wireless sensor using a flex-circuit inductive transducer. Sens. Actuators A 2009, 155, 58–65. [Google Scholar] [CrossRef]
- Sun, H.; Almdal, K.; Andresen, T.L. Expanding the dynamic measurement range for polymeric nanoparticle pH sensors. Chem. Commun. 2011, 47, 5268–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Lotsch, B.V. Stimuli-responsive 2D polyelectrolyte photonic crystals for optically encoded pH sensing. Chem. Commun. 2012, 48, 6169–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Li, F.; Zhu, Y.; Shen, J. The switch-type humidity sensing properties of polyacrylic acid and its copolymers. J. Mater. Sci. 2000, 35, 2005–2008. [Google Scholar] [CrossRef]
- Barry, R.A.; Wiltzius, P. Humidity-sensing inverse opal hydrogels. Langmuir 2006, 22, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Arregui, F.J.; Ciaurriz, Z.; Oneca, M.; Matias, I.R. An experimental study about hydrogels for the fabrication of optical fiber humidity sensors. Sens. Actuators B 2003, 96, 165–172. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ahmad, U.; Rajesh, K.; Hamed, A.; Al-Assir, M.S. Poly(acrylic acid)/multi-walled carbon nanotube composites: Efficient scaffold for highly sensitive 2-nitrophenol chemical censor. Nanosci. Nanotechnol. Lett. 2016, 8, 200–206. [Google Scholar] [CrossRef]
- Han, D.-M.; Zhang, Q.M.; Serpe, M.J. Poly (N-isopropylacrylamide)-co-(acrylic acid) microgel/Ag nanoparticle hybrids for the colorimetric sensing of H2O2. Nanoscale 2015, 7, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Y.; Seshadri, P.; Torsi, L.; Manoli, K.; Mallardi, A.; Ditaranto, N.; Scantacroce, M.V.; Di Franco, C.; Scamarcio, G.; Magliulo, M. UV crosslinked poly(acrylic acid): A simple method to bio-functionalize electrolyte-gated OFET biosensors. J. Mater. Chem. B 2015, 3, 5049–5057. [Google Scholar] [CrossRef]
- Van der Linden, H.J.; Herber, S.; Olthuis, W.; Bergveld, P. Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst 2003, 128, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, A.; Leal, M.P.; Deka, S.R.; George, C.; Franchini, I.R.; Falqui, A.; Cingolani, R.; Pellegrino, T. “Nanohybrids” based on pH-responsive hydrogels and inorganic nanoparticles for drug delivery and densor applications. Nano Lett. 2011, 11, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; Kuckling, D.; Corten, C.; Gerlach, G.; Sorber, J.; Suchaneck, G.; Amdt, K.F. Chemical sensors based on multiresponsive block copolymer hydrogels. Sens. Actuators B 2007, 126, 97–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.F.; Brown, G.M.; Thundat, T. Detection of CrO42− using a hydrogel swelling microcantilever sensor. Anal. Chem. 2003, 75, 4773–4777. [Google Scholar] [CrossRef] [PubMed]
- Herber, S.; Olthuis, W.; Bergveld, P.; van den Berg, A. Exploitation of a pH-sensitive hydrogel disk for CO2 detection. Sens. Actuators B 2004, 103, 284–289. [Google Scholar] [CrossRef]
- Huang, W.-D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Lin, G.; Chang, S.; Kuo, C.; Magda, J.; Solzbacher, F. Free swelling and confined smart hydrogels for applications in chemomechanical sensors for physiological monitoring. Sens. Actuators B 2009, 136, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Rich, R.L.; Papalia, G.A.; Flynn, P.J.; Furneisen, J.; Quinn, J.; Klein, J.S.; Katsamba, P.S.; Waddell, M.B.; Scott, M.; Thompson, J.; et al. A Global Benchmark study using affinity-based biosensors. Anal. Biochem. 2009, 386, 194–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-L.; Xu, J.J.; Du, Y.; Chen, H.Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mousty, C.; Lepellec, A.; Cosnier, S.; Novoa, A.; Marks, R.S. Fabrication of organic phase biosensors based on multilayered polyphenol oxidase protected by an alginate coating. Electrochem. Commun. 2001, 3, 727–732. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Bao, J.; Tu, W.; Dai, Z. A superoxide anion biosensor based on direct electron transfer of superoxide dismutase on sodium alginate sol–gel film and its application to monitoring of living cells. Anal. Chim. Acta 2012, 717, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.-P.; Leroux, F.; Mousty, C. Amperometric biosensors based on LDH-ALGINATE hybrid nanocomposite for aqueous and non-aqueous phenolic compounds detection. Sens. Actuators B 2010, 150, 36–42. [Google Scholar] [CrossRef]
- Joshi, A.; Solanki, S.; Chaudhari, R.; Bahadur, D.; Aslam, M.; Sirvastava, R. Multifunctional alginate microspheres for biosensing, drug delivery and magnetic resonance imaging. Acta Biomater. 2011, 7, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Polyak, B.; Geresh, S.; Marks, R.S. Synthesis and characterization of a biotin-alginate conjugate and its application in a biosensor construction. Biomacromolecules 2004, 5, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Joshi, A.; Jayant, R.D.; Srivastava, R. Cholesterol biosensors based on oxygen sensing alginate-silica microspheres. Biotechnol. Bioeng. 2011, 108, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Novoa, A.; Mousty, C.; Marks, R.S. Biotinylated alginate immobilization matrix in the construction of an amperometric biosensor: Application for the determination of glucose. Anal. Chim. Acta 2002, 453, 71–79. [Google Scholar] [CrossRef]
- Chico, B.; Camacho, C.; Perez, M.; Longo, M.A.; Sanroman, M.A.; Pingarron, J.M.; Villalonga, R. Polyelectrostatic immobilization of gold nanoparticles-modified peroxidase on alginate-coated gold electrode for mediatorless biosensor construction. J. Electroanal. Chem. 2009, 629, 126–132. [Google Scholar] [CrossRef]
- Abu-Rabeah, K.; Polyak, B.; lonescu, R.E.; Cosnier, S.; Marks, R.S. Synthesis and characterization of a pyrrole-alginate conjugate and its application in a biosensor construction. Biomacromolecules 2005, 6, 3313–3318. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Zhang, H.; Duan, X.; Xu, J.; Wen, Y. Electrochemical sensing application of poly(acrylic acid modified EDOT-co-EDOT):PSS and its inorganic nanocomposite with high soaking stability, adhesion ability and flexibility. RSC Adv. 2015, 5, 12237–12247. [Google Scholar] [CrossRef]
- Homma, T.; Sumita, D.; Kondo, M.; Kuwahara, T.; Shimomura, M. Amperometric glucose sensing with polyaniline/poly(acrylic acid) composite film bearing covalently-immobilized glucose oxidase: A novel method combining enzymatic glucose oxidation and cathodic O2 reduction. J. Electroanal. Chem. 2014, 712, 119–123. [Google Scholar] [CrossRef]
- Yang, M.; Su, F.; Dong, X.; Ma, C.; Pang, L.; Peng, D.; Wang, M.; He, L.; Zhang, Z. Hierarchical porous microspheres of the Co3O4@graphene with enhanced electrocatalytic performance for electrochemical biosensors. Biosens. Bioelectron. 2017, 89, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Su, F.; Dong, X.; Ma, C.; Pang, L.; Peng, D.; Wang, M.; He, L.; Zhang, Z. Development of glucose biosensors based on plasma polymerization-assisted nanocomposites of polyaniline, tin oxide, and three-dimensional reduced graphene oxide. Appl. Surf. Sci. 2017, 401, 262–270. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Xie, Y.; Yu, J.; Yang, H.; Miao, L.; Song, Y. Three-dimensional macroporous carbon/hierarchical Co3O4 nanoclusters for nonenzymatic electrochemical glucose sensor. Appl. Surf. Sci. 2017, 402, 47–52. [Google Scholar] [CrossRef]
- Ko, T.-H.; Radhakrishnam, S.; Seo, M.K.; Khil, M.S.; Kim, H.Y.; Kim, B.S. A green and scalable dry synthesis of NiCo2O4/graphene nanohybrids for high-performance supercapacitor and enzymeless glucose biosensor applications. J. Alloys Compd. 2017, 696, 193–200. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Rabiee, M.; Abdolrahim, M.; Tahiri, M.; Vashaee, D.; Tayebi, L. A novel electrochemical biosensor based on Fe3O4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose. Anal. Biochem. 2017, 519, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Wang, D.; Qiao, Z.; Qu, L. Synthesis of nanoneedle-like copper oxide on N-doped reduced graphene oxide: A three-dimensional hybrid for nonenzymatic glucose sensor. Sens. Actuators B 2017, 238, 588–595. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Peltola, E.; Peralta, D.; Igarzabal, C.I.A.; Baruzzi, A.M.; Strumia, M.C.; Garay, F. Nanodiamonds on tetrahedral amorphous carbon significantly enhance dopamine detection and cell viability. Biosens. Bioelectron. 2017, 88, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Peralta, D.; Alvarez Igarzabal, C.I.; Baruzzi, A.M.; Strumia, M.C.; Garay, F. Supramolecular complex based on MWNTs/Boltorn H40 provides fast response to a Sandwich-type amperometric lactate biosensor. Sens. Actuators B 2017, 244, 577–584. [Google Scholar] [CrossRef]
- Dagar, K.; Pundir, C.S. An improved amperometric l-lactate biosensor based on covalent immobilization of microbial lactate oxidase onto carboxylated multiwalled carbon nanotubes/copper nanoparticles/polyaniline modified pencil graphite electrode. Enzyme Microb. Technol. 2017, 96, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.; Kong, Q.; Luo, X.; Jiao, K. Toward DNA electrochemical sensing by free-standing ZnO nanosheets grown on 2D thin-layered MoS2. Biosens. Bioelectron. 2017, 89, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, H.; Chen, X.; Lu, M. Low-temperature thermal reduction of suspended graphene oxide film for electrical sensing of DNA-hybridization. Mater. Sci. Eng. C 2017, 72, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Brink, R.; Nandi, D.; Siwal, S.; Mallick, K. Gold nanoparticle within the polymer chain, a multi-functional composite material, for the electrochemical detection of dopamine and the hydrogen atom-mediated reduction of Rhodamine-B, a mechanistic approach. J. Mater. Sci. 2017, 52, 770–781. [Google Scholar] [CrossRef]
- Fandrich, A.; Buller, J.; Memczak, H.; Stocklein, W.; Hinrichs, K.; Wischerhoff, E.; Schulz, B.; Laschewsky, A.; Lisdat, F. Responsive polymer-electrode interface-study of its thermo- and pH-sensitivity and the influence of peptide coupling. Electrochim. Acta 2017, 229, 325–333. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Ma, Z. Conductive hydrogel composed of 1,3,5-benzenetricarboxylic acid and Fe3+ used as enhanced electrochemical immunosensing substrate for tumor biomarker. Bioelectrochemtry 2017, 114, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; Lin, Y.; de la Guardia, M. Nanomaterials for use in immunosensing of carcinoembryonic antigen (CEA): Recent advances. Trends Analyt. Chem. 2017, 86, 185–205. [Google Scholar] [CrossRef]
- Torres, D.I.; Miranda, M.V.; Campo Dall’ Orto, V. One-pot preparation of SBP-PANI-PAA-ethylene glycol diglycidyl ether sensor for electrochemical detection of H2O2. Sens. Actuators B 2017, 239, 1016–1025. [Google Scholar] [CrossRef]
- Bjerketorp, J.; Hakansson, S.; Belkin, S.; Jansson, J.K. Advances in preservation methods: Keeping biosensor microorganisms alive and active. Curr. Opin. Biotechnol. 2006, 17, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Bio-smart hydrogels: Co-joined molecular recognition and signal transduction in biosensor fabrication and drug delivery. Biosens. Bioelectron. 2002, 17, 973–981. [Google Scholar] [CrossRef]
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pogorelova, S.P.; Bourenko, T.; Kharitonov, A.B.; Willner, I. Selective sensing of triazine herbicides in imprinted membranes using ion-sensitive field-effect transistors and microgravimetric quartz crystal microbalance measurements. Analyst 2002, 127, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Sallacan, N.; Zayats, M.; Bourenko, T.; Kharitonov, A.B.; Willner, I. Imprinting of nucleotide and monosaccharide recognition sites in acrylamidephenylboronic acid-acrylamide copolymer membranes associated with electronic transducers. Anal. Chem. 2002, 74, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Barriet, D.; Sletmoen, M.; Stokke, B.T. Responsive hydrogels for label-free signal transduction within biosensors. Sensors 2010, 10, 4381–4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, T.H.; Shuler, M.L. Integration of cell culture and microfabrication technology. Biotechnol. Prog. 2003, 19, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Troyer, K.P.; Heien, M.L.; Venton, B.J.; Wightman, R.M. Neurochemistry and electroanalytical probes. Curr. Opin. Chem. Biol. 2002, 6, 696–703. [Google Scholar] [CrossRef]
- Venton, B.J.; Troyer, K.P.; Wightman, R.M. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Anal. Chem. 2002, 74, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Wightman, R.M. Detection technologies. Probing cellular chemistry in biological systems with microelectrodes. Science 2006, 311, 1570–1574. [Google Scholar] [PubMed]
- Jung, S.-K.; Kauri, L.M.; Qian, W.J.; Kennedy, R.T. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca2+ in single islets of langerhans. J. Biol. Chem. 2000, 275, 6642–6650. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, Y.; Zhu, H.; Marcu, L.; Revzin, A. Enzyme-containing hydrogel micropatterns serving a dual purpose of cell sequestration and metabolite detection. Biosens. Bioelectron. 2009, 24, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Klauke, N.; Glidle, A.; Cobbold, P.; Smith, G.L.; Cooper, J.M. Ultra-low-volume, real-time measurements of lactate from the single heart cell using microsystems technology. Anal. Chem. 2002, 74, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Kauri, L.M.; Jung, S.-K.; Kennedy, R.T. Direct measurement of glucose gradients and mass transport within islets of Langerhans. Biochem. Biophys. Res. Commun. 2003, 304, 371–377. [Google Scholar] [CrossRef]
- Cheng, W.; Klauke, N.; Sedgwick, H.; Smith, G.L.; Cooper, J.M. Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform. Lab Chip 2006, 6, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Zguris, J.; Pishko, M.V. Nitric oxide sensitive fluorescent poly(ethylene glycol) hydrogel microstructures. Sens. Actuators B 2006, 115, 503–509. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Recco, L.C.; Delella, F.K.; Pedrosa, V.A. A novel superoxide anion biosensor for monitoring reactive species of oxygen released by cancer cells. Electroanalysis 2017, 29. [Google Scholar] [CrossRef]
- Pal, R.K.; Kundu, S.C.; Yadavalli, V.K. Biosensing using photolithographically micropatterned electrodes of PEDOT:PSS on ITO substrates. Sens. Actuators B 2017, 242, 140–147. [Google Scholar] [CrossRef]
- Tria, S.A.; Lopez-Ferber, D.; Gonzalez, C.; Bazin, I.; Guiseppi-Elie, A. Microfabricated biosensor for the simultaneous amperometric and luminescence detection and monitoring of Ochratoxin A. Biosens. Bioelectron. 2016, 79, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminshi, Y.; Kieninger, J.; Urban, G.A. Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip 2014, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, P.; Määttänen, A.; Sandler, N. Printing technologies for biomolecule and cell-based applications. Int. J. Pharm. 2015, 494, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lifson, M.A.; Carter, J.A.; Miller, B.L. Functionalized polymer microgel particles enable customizable production of label-free sensor arrays. Anal. Chem. 2015, 87, 7887–7893. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Itabashi, A.; Wagner, T.; Schoning, M.J.; Yoshinobu, T. High-speed chemical imaging inside a microfluidic channel. Sens. Actuators B 2014, 194, 521–527. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Lin, J.-M. Microfluidic technologies in cell isolation and analysis for biomedical applications. Analyst 2017, 142, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, Y.; Pan, L.; Shi, Y.; Yu, G. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2920–2930. [Google Scholar] [CrossRef]
- Strakosas, X.; Huerta, M.; Donahue, M.J.; Hama, A.; Pappa, A.M.; Ferro, M.; Ramuz, M.; Rivnay, J.; Owens, R.M. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.Y.C.; Lin, W.C. Optical ATP biosensor for extracellular ATP measurement. Biosens. Bioelectron. 2013, 43, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Wells, M.C.; van der Meer, J.R. Whole-cell living biosensors—are they ready for environmental application? Appl. Microb. Biotechnol. 2006, 70, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Roger, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Llaudet, E.; Hatz, S.; Droniou, M.; Dale, N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal. Chem. 2005, 77, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Lilienthal, S.; Kahn, J.S.; Riutin, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017, 8, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhao, Z.; Lv, Y.F.; Huan, S.Y.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. DNAzyme-based biosensors and nanodevices. Chem. Commun. 2015, 51, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, D.C.; Clark, G.; Cannon, A.E.; Roux, S.; Chaturvedi, P.; McLamore, E.S. A self-referencing biosensor for real-time monitoring of physiological ATP transport in plant systems. Biosens. Bioelectron. 2015, 74, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Nurunnabi, M.; Norshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.; Jaffa, A.A. Application of biosensors in tissue engineering. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, S.N.; Aydinlik, S.; Aladag, N.; Ozsoz, M.; Ozkan-Ariksoysal, D. Different DNA immobilization strategies for the interaction of anticancer drug irinotecan with DNA based on electrochemical DNA biosensors. Comb. Chem. High Throughput Screen. 2010, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Budnikov, H.C.; Evtugyn, G.A.; Porfireva, A.V. Electrochemical DNA sensors based on electropolymerized materials. Talanta 2012, 102, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, A.; Nishino, T.; Maruyama, T. Display of amino groups on substrate surfaces by simple dip-coating of methacrylate-based polymers and its application to DNA immobilization. Langmuir 2013, 29, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, S.N. Gelatin methacrylate (GelMA) mediated electrochemical DNA biosensor for DNA hybridization. Biosens. Bioelectron. 2015, 64, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Biela, A.; Watkinson, M.; Meier, U.C.; Baker, D.; Giovannoni, G.; Becer, C.R.; Krause, S. Disposable MMP-9 sensor based on the degradation of peptide cross-linked hydrogel films using electrochemical impedance spectroscopy. Biosens. Bioelectron. 2015, 68, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Rio, J.; Espejo, C.; de Villa, M.R.; Al-zayat, H.; Nos, C.; Deisenhammer, F.; Baranzini, S.E.; Nonell, L.; Lopez, C.; et al. Changes in matrix metalloproteinases and their inhibitors during interferon-beta treatment in multiple sclerosis. Clin. Immunol. 2009, 130, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Bar-Shir, A.; Song, X.; Gilad, A.A.; Walczak, P.; Bulte, J.W.M. Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials 2015, 42, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Urmann, K.; Walter, J.G.; Scheper, T.; Segal, E. Label-free optical biosensors based on aptamer-functionalized porous silicon scaffolds. Anal. Chem. 2015, 87, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Bampton, E.T.; Goemans, C.G.; Niranjan, D.; Mizushima, N.; Tolkovsky, A.M. The dynamics of autophagy visualized in live cells: From autophagosome formation to fusion with endo/lysosomes. Autophagy 2005, 1, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Dini, V.; Francesco, F.D.; Romanelli, M. The role of biomedical sensors in wound healing. Wound Med. 2015, 8, 15–18. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.D.; Landthaler, M.; Babilas, P.; Wofbeis, O.S. Simultaneous photographing of oxygen and pH in vivo using sensor films. Angew. Chem. Int. Ed. Engl. 2011, 50, 10893–10896. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, D.A.; Bannwarth, M.B.; Schulenburg, S.; Faccio, G.; Maniura-Weber, K.; Rossi, R.M.; Scherer, L.; Richter, M.; Boesel, L.F. Simultaneous detection of pH value and glucose concentrations for wound monitoring applications. Biosens. Bioelectron. 2017, 87, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Ochoa, M.; Parupudi, T.; Zhao, X.; Yazdi, I.K.; Dokmeci, M.R.; Tamayol, A.; Khademhosseini, A.; Ziaie, B. A low-cost flexible pH sensor array for wound assessment. Sens. Actuators B 2016, 229, 609–617. [Google Scholar] [CrossRef]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Khan, M.A.; Ansari, U.; Ali, M.N. Real-time wound management through integrated pH sensors: A review. Sens. Rev. 2015, 35, 183–189. [Google Scholar] [CrossRef]
- Heinzle, A.; Papen-Botterhuis, N.E.; Schiffer, D.; Schneide, K.P.; Binder, B.; Schintler, M.; Haaksman, L.K.; Lenting, H.B.; Gubitz, G.M.; Sigl, E. Novel protease-based diagnostic devices for detection of wound infection. Wound Repair Regen. 2013, 21, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Boelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, M.D.; Kassal, P.; Steinberg, I.M. System architectures in wearable electrochemical sensors. Electroanalysis 2016, 28, 1149–1169. [Google Scholar] [CrossRef]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New developments in smart bandage technologies for wound diagnostics. Adv. Mater. 2016, 28, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef]
- Ngoepe, M.; Choonara, Y.E.; Tyagi, C.; Tomar, L.K.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Integration of biosensors and drug delivery technologies for early detection and chronic management of illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.; Phan, Q.T.; El-Sharif, H.; Govada, L.; Stevenson, D.; Chayen, N.E. Protein crystallization and biosensor applications of hydrogel-based molecularly imprinted polymers. Biomacromolecules 2012, 13, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Kang, H.; Donovan, M.; Zhu, Z.; Tan, W. Aptamer-incorporated hydrogels for visual detection, controlled drug release, and targeted cancer therapy. Anal. Bioanal. Chem. 2012, 402, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; Yang, M.; Mak, A. Nanoporous membrane-based cell chip for the study of anti-cancer drug effect of retinoic acid with impedance spectroscopy. Talanta 2009, 80, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Stegbauer, K.; Wolf, B. Monitoring of cellular behaviour by impedance measurements on interdigitated electrode structures. Biosens. Bioelectron. 1997, 12, 29–41. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, J.; Xiao, L.; Tang, J.C.O.; Zhang, Y.; Wang, P.; Yang, M. Impedance studies of bio-behavior and chemosensitivity of cancer cells by micro-electrode arrays. Biosens. Bioelectron. 2009, 24, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenzavirus. Biosens. Bioelectron. 2013, 42, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Yurke, B.; Langrana, N.A. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng. 2004, 126, 104–110. [Google Scholar] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbah, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ozawa, S.; Okuda, N.; Yanagid, Y.; Tanaka, S.; Hatsuzawa, T. Reflectometric detection of influenza virus in human saliva using nanoimprint lithography-based flexible two-dimensional photonic crystal biosensor. Sens. Actuators B 2010, 148, 269–276. [Google Scholar] [CrossRef]
- Lee, N.Y.; Jung, Y.K.; Park, H.G. On-chip colorimetric biosensor based on polydiacetylene (PDA) embedded in photopolymerized poly(ethylene glycol) diacrylate (PEG-DA) hydrogel. Biochem. Eng. J. 2006, 29, 103–108. [Google Scholar] [CrossRef]
- Pang, Y.; Rong, Z.; Wang, J.; Xiao, R.; Wang, S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core–shell nanoparticles metal-enhanced fluorescence (MEF). Biosens. Bioelectron. 2015, 66, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, B.T.T.; Koh, G.; Lim, H.S.; Chua, A.J.S.; Ng, M.M.L.; Toh, C.S. Membrane-based electrochemical nanobiosensor for the detection of virus. Anal. Chem. 2009, 81, 7226–7234. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Yoo, J.E.; Tarver, J.; Loo, Y.L.; Heller, A. An electron-conducting cross-linked polyaniline-based redox hydrogel, formed in one step at pH 7.2, wires glucose oxidase. J. Am. Chem. Soc. 2007, 129, 7006–7007. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Pishko, M.V. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal. Chem. 1999, 71, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, B.; Deng, Q.; Dong, S. Amperometric glucose biosensor based on sol–gel organic–inorganic hybrid material. Anal. Chem. 1998, 70, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-D.; Huang, J.; Liang, R.-P. Nanocomposite film based on graphene oxide for high performance flexible glucose biosensor. Sens. Actuators B 2011, 160, 287–294. [Google Scholar] [CrossRef]
- Reiter, S.; Habermüller, K.; Schuhmann, W. A reagentless glucose biosensor based on glucose oxidase entrapped into osmium-complex modified polypyrrole films. Sens. Actuators B 2001, 79, 150–156. [Google Scholar] [CrossRef]
- Shibata, H.; Heo, Y.J.; Okisu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ikeda, R.; Yanagida, Y.; Hatsuzawa, T. Stimuli-responsive hydrogel–silver nanoparticles composite for development of localized surface plasmon resonance-based optical biosensor. Anal. Chim. Acta 2008, 611, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bornhoeft, L.; Biswas, A.; McShane, M. Composite hydrogels with engineered microdomains for optical glucose sensing at low oxygen conditions. Biosensors 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kajisa, T.; Sakata, T. Glucose-responsive hydrogel electrode for biocompatible glucose transistor. Sci. Technol. Adv. Mater. 2017, 18, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.C.; Hon, P.Y.; Mak, K.K.; Renneberg, R. Amperometric determination of lactate with novel trienzyme/poly(carbamoyl) sulfonate hydrogel-based sensor. Biosens. Bioelectron. 2004, 19, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Garay, F.; Baruzzi, A.M. Design and optimization of a lactate amperometric biosensor based on lactate oxidase cross-linked with polymeric matrixes. Sens. Actuators B 2008, 131, 590–595. [Google Scholar] [CrossRef]
- Zanini, V.P.; López de Mishima, B.; Solís, V. An amperometric biosensor based on lactate oxidase immobilized in laponite–chitosan hydrogel on a glassy carbon electrode. Application to the analysis of l-lactate in food samples. Sens. Actuators B 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Zhybak, M.; Beni, V.; Vagin, M.Y.; Dempsey, E.; Tumer, A.P.F.; Korpan, Y. Creatinine and urea biosensors based on a novel ammonium ion-selective copper-polyaniline nano-composite. Biosens. Bioelectron. 2016, 77, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Sarkar, P. Enzymatic electrochemical biosensor for urea with a polyaniline grafted conducting hydrogel composite modified electrode. RSC Adv. 2016, 6, 92520–92533. [Google Scholar] [CrossRef]
- Ma, W.J.; Luo, C.H.; Lin, J.L.; Chou, S.H.; Chen, P.H.; Syu, M.J.; Kuo, S.H.; Lai, S.C. A portable low-power acquisition system with a urease bioelectrochemical sensor for potentiometric detection of urea concentrations. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Li, X.; Kim, J.; Lim, B.O.; Saleh Ahammad, A.J.; Lee, J.J. A cholesterol biosensor based on a bi-enzyme immobilized on conducting poly(thionine) film. Sens. Actuators B 2014, 202, 536–542. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Amperometric determination of cholesterol in serum using a biosensor of cholesterol oxidase contained within a polypyrrole–hydrogel membrane. Anal. Chim. Acta 2001, 448, 27–36. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Deluca, G.; Bulko, T.; Carrara, S.; Nicolini, C.; Usanov, S.A.; Archakov, A. Cholesterol amperometric biosensor based on cytochrome P450scc. Biosens. Bioelectron. 2004, 19, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Solanki, P.R.; Pandey, M.K.; Malhotra, B.D. Cholesterol biosensor based on cholesterol esterase, cholesterol oxidase and peroxidase immobilized onto conducting polyaniline films. Sens. Actuators B 2006, 115, 534–541. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Xu, S.; Chen, L.; Zhou, N.; Xiong, H.; Peng, H. Label-free colorimetric detection of trace cholesterol based on molecularly imprinted photonic hydrogels. J. Mater. Chem. 2011, 21, 19267–19274. [Google Scholar] [CrossRef]




| Bioreceptor | Advantage | Disadvantage |
|---|---|---|
| Antibody [13,14,45] | The immunogen need not be purified prior to detection. | Expensive and time-consuming method. Miniaturized immune-PCR detection methods have not yet been commercialized. |
| Enzymes [17,18,46,47,48] | Variety of reaction products arising from the catalytic process. | Stability problems have been reported. The detection limits can be very low due to signal amplification. |
| Nucleic acids [49,50] | Target molecule can be recognized by shape and sequence. A wide range of biomolecules can be detected. High binding affinity, simple synthesis method and easy storage have been reported. | It is not easy to design donor/acceptor labeling strategies. They are sensitive to pyrimidine specific nucleases that are abundant in biofluids. |
| Cells or cellular structures [7,26,29,31] | Can be used over prolonged periods of time as cells are closed systems. | |
| Biomimetic [32,33,34,35,36,37] | Known as an effective, accessible and inexpensive strategy. Physically, very stable (solid-like). | Molecular imprint probes do not have the same flexibility and selectivity as actual bioreceptors. |
| The molecular imprinted polymers can survive in destructive environments. |
| Glucose | |||
|---|---|---|---|
| Hydrogel | Transduction Strategy | Technical Specification | Ref. |
| Polyaniline | Electrochemical | Sensitivity = 96.1 μA·mM−1·cm−2 | [248] |
| Response time = 3 s | |||
| Linear range = 0.01–8 mM | |||
| Polyaniline-PEG | Electrochemical | N/A | [249] |
| PEG | Optical | Linear range = 0–600 mg/dL | [250] |
| Response time = 10 min | |||
| PVA-Vinyl pyridine | Electrochemical | Sensitivity = 600 nA·mM−1·L−1 | [251] |
| Response time = 11 s | |||
| Chitosan | Electrochemical | Linear range = 5 μM–2.5 mM | [146] |
| Response time = 7 s | |||
| Chitosan-graphene oxide | Electrochemical | Linear range = 0.02–6.78 mM | [252] |
| Sensitivity = 10 μA·mM−1·cm−2 | |||
| Polypyrrole | Electrochemical | Linear range = up to 15 mM | [253] |
| PEG (injectable) | Optical | Linear range = up to 370 mg·dL−1 | [254] |
| Response time = 11 min | |||
| Polyvinylpyrrolidone | Optical | N/A | [255] |
| Alginate | Optical | Sensitivity = 0.80 ± 0.11 μs·dL·mg−1 | [256] |
| Linear range = 2.6–350 mg/dL | |||
| HEMA | Electrochemical | Linear range = 10 μM–40 mM | [257] |
| Lactate | |||
| BH40 (Hyper-branched) | Electrochemical | Response time = 7 s | [167] |
| Linear range = up to 580 mg/L | |||
| Polycarbamoyl sulfonate | Electrochemical | Response time = 2 s | [258] |
| Linear range = 10–400 μM | |||
| Albumin-mucin | Electrochemical | Response time = 90 s | [259] |
| Linear range = 0.7 μM–1.5 mM | |||
| Chitosan | Electrochemical | Sensitivity = 0.32 A·M−1·cm−2 | [260] |
| Response time = 5 s | |||
| Urea | |||
| Polyaniline | Electrochemical | Sensitivity = 85 mA·M−1·cm−2 | [261] |
| Response time = 15 s | |||
| Poly aniline | Electrochemical | Sensitivity = 878 μA·M−1·cm−2 | [262] |
| Aniline-co-o-phenylenediamine | Electrochemical | sensitivity = 31.12 mV/log [M] | [263] |
| Linear range = 3.16 × 10−4–3.16 × 10−2 M | |||
| Cholesterol | |||
| Poly(thionine) | Electrochemical | Linear range = 25–125 μM | [264] |
| Sensitivity = 0.18 μA·mM−1·cm−2 | |||
| Polypyrrole | Electrochemical | Linear range = 5 × 10−4–1.5 × 10−2 M | [265] |
| Response time = 30 s | |||
| Agarose | Electrochemical | Sensitivity = 6.9 nA·μM−1 | [266] |
| Response time = 120 s | |||
| Polyaniline | Electrochemical | Sensitivity = 0.042 μA·mg·dL−1 | [267] |
| Response time = 240 s | |||
| Polymethacrylate | Optical | Response time = 120 s | [268] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9080364
Tavakoli J, Tang Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers. 2017; 9(8):364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9080364
Chicago/Turabian StyleTavakoli, Javad, and Youhong Tang. 2017. "Hydrogel Based Sensors for Biomedical Applications: An Updated Review" Polymers 9, no. 8: 364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym9080364