1. Introduction
In the future decades, the transition from a fossil-based to a biomass-based economy is crucial for economic and environmental sustainability; therefore, Europe aims at achieving an optimal and efficient multipurpose exploitation of renewable biomasses as food, added valued chemicals and energy [
1,
2,
3]. As well, the impact in biomass production for the bioeconomy is crucial for environmental sustainability [
4,
5]. Land degradation caused by human activities negatively impacts the well-being of at least 3.2 billion people, costing more than 10% of annual global gross products through the loss of biodiversity and ecosystem services [
6]. Soil degradation, as part of land degradation, includes the loss of soil through erosion and the depletion of soil organic matter (SOM) [
3,
7]; it has been estimated that nearly two billion ha (23%) of the world’s used lands— agricultural lands, permanent pastures, forests and woodlands—have been degraded since the mid-twentieth century [
8], causing important implications, particularly in the Mediterranean area [
9]. Soil organic Carbon (SOC), conventionally assumed to be 58% of the SOM [
10], is beneficial for soil health for many reasons, including improved fertility, productivity and soil biodiversity, reduced erosion, runoff and water pollution, and can help buffer crops and pasture systems against the impacts of climate change [
11,
12]. Furthermore, maintaining organic C-rich soils, restoring and improving degraded agricultural lands and, in general terms, increasing the soil C could play fundamental roles in addressing food security and in mitigating the anthropogenic greenhouse gas (GHG) emissions. For these reasons, any bio-based product chain should start from a responsible use of agricultural land, such as a sustainable SOC management. Due to the high variability of SOC and slow change, the time horizon is usually referred almost to 20 years and dynamics assessed by modeling. Within this context, the assessment of the biorefinery cropping system on the SOC stock (SOCS) variation over a long period becomes a key factor. To this end, the Horizon 2020 First2Run project (
http://www.first2run.eu/) provided soil and crop data measured in three farm trials under
Cynara cardunculus L. (CAR). This project aimed at demonstrating the techno, economical and environmental sustainability at the industrial scale of a first-of-its-kind value chain with a low input and underutilized oil crop (i.e., CAR) grown in arid and/or marginal lands whose vegetable oil is converted into bio-monomers as building blocks for high added value bio-based products, i.e., biolubricants, cosmetics, bioplastics and additives, through the integration of chemical and biotechnological processes [
13]. Furthermore, several authors showed how simple cropping techniques and low production costs are the main requirements to produce nonfood crops [
14], and the growth of perennial crops, as CAR, increase the amount of SOC sequestration in soil compared with annual arable cropping systems [
15,
16].
In the last 80 years, several process-oriented models have been developed to predict SOC dynamics and biogeochemical-related soil processes on a daily, monthly or annual basis [
17,
18]. At sites where less-detailed data are available, models requiring few parameters able to produce reliable and accurate simulations are preferred in comparison with those requiring large amounts of data (meteorological data, crop phenological data, soil chemical and physical characteristics), which can limit their use. In this context, among the simplest models used for assessing SOC dynamics, the 20-year step Intergovernmental Panel on Climate Change (IPCC) tool [
19,
20], the annual-step humic balance family [
21,
22,
23] such as the SOM stock dynamics for the Biorefinery Integrated into the Territory (SOMBIT) model [
24] and the monthly-step Rothamsted carbon (RothC) model [
25] have been extensively applied to numerous field studies worldwide to predict the SOC dynamics under various types of agricultural management and agroclimatic regions (e.g., the IPCC tool: [
26,
27,
28,
29]; humic balance: [
30,
31,
32] and RothC: [
33,
34,
35]). Both humic balance and RothC models follow exponential kinetics and reflect SOC variations over 1- to 100-year periods well.
In the Mediterranean region, these models and revised versions have already been used both at the plot (e.g., [
36,
37,
38,
39]) and sub-regional (e.g., [
40,
41]) scales.
However, to the best of our knowledge, there have not been studies so far investigating SOC dynamics at the plot scale, linking CAR cultivation with prediction management scenarios using different modeling approaches. Thereby, the main general aim of this study was to evaluate how the introduction of perennial CAR in a typical industrial rainfed cropping system of Sardinia (Italy) influenced the SOCS dynamic and sequestration in the topsoil (0–30 cm) over a 20-year period (2012–2031) by using the IPCC tool, SOMBIT and RothC models. This study focused on quantifying how this cropping system could contribute in three Mediterranean marginal lands to mitigate climate change.
4. Discussion
To the best of our knowledge, this paper is the first study in the Mediterranean Basin assessing the influence of CAR crop introduction in a typical industrial rainfed cropping system on SOCS change in a 20-year scenario perspective using a modeling approach.
Several studies have indicated that CAR—native to the Mediterranean Basin—is one of the most promising species for energy production in this area [
73,
74,
75]. The crop characteristics that support these applications are the relatively low crop input and large biomass productivity, mainly of lignocellulosic compositions and high heating values [
76].
Moreover, the introduction of these crops into the traditional agricultural system may represent an interesting alternative to increase the agroenvironmental cropping system’s sustainability. This is because CAR directly enhances the soil fertility due to the reduction of the tsoil tillage frequency and low nutritional requirements [
77] and reduces the soil degradation due to the protection of its dense canopy against the erosion [
78,
79].
CAR is a perennial herbaceous rhizomatous root species native to the Mediterranean Basin [
14,
80], which can grow up to 2-m-high and presents the leaves of the basal rosette petiolata very large and coriaceous [
81]. It is an autumn-sown and summer-harvested crop, with an annual reproductive cycle, which is completed yearly by the end of spring [
82].
In Mediterranean conditions, it is normally rainfed, and the biomass depends only on the amount of the rainwater accumulated during autumn, winter and early spring in the soil and extracted by its deep roots system [
83]. Good performances occur with 450-mm water availability in the period ranging from shoot emissions to head differentiations [
76]. The CAR biomass yields observed in the present study (
Table 4) are comparable with the scientific literature available from similar studies carried out in other rainfed Mediterranean areas. In fact, the aboveground biomass production during the first growth year is usually low; then, the biomass production usually rises to the third and becomes more stable over the following years [
84], decreasing by the seventh or subsequent years [
77,
85]. In this research, the average production in grain and straws was lower in YR1 and higher in YR2–6, while, in YR7, decreased slightly, hence corroborating the planning of a seven-year CAR cultivation also in the study area. Further, in a study carried out in the Basilicata Region (Southern Italy), Piscioneri et al. [
86] observed a three-year average dry biomass yield of 12.5 Mg ha
−1 (ranging from 10.0 to 15.0 Mg ha
−1). Similarly, Ledda et al. [
87] in Sardinia (Italy) observed a dry biomass yield of 10.2 Mg ha
−1 (ranging from 6.8 to 11.6 Mg ha
−1), while Francaviglia et al. [
88] and Neri et al. [
79] in a marginal hilly area of the Latium Region (Central Italy) reported a yield of 12.9 and 11.4 Mg ha
−1 as the average, respectively. In Portugal, Gominho et al. [
89] reported a total dry biomass yield of 9.7 Mg ha
−1 (range 4.4–18.4 Mg ha
−1), while in Northern Greece, Vasilakoglou and Dhima [
90] observed dry biomass yields of 11.0 Mg ha
−1.
Since the cropping system’s purpose aimed to maintain or increase the SOCS, in both scenarios, straws of FB and DW after the harvests were supposed to be left on the ground and incorporated by the next tillage. Indeed, in the study area, in 70% of the cropping systems, straw is left in the field [
91], notably in marginal areas where yields are lower. CAR straws were harvested as biorefinery feedstock, although 30% of the aboveground biomass was left in the soil to increase sustainability, instead of 10%, as reported in previous trials [
24].
The models required specific parametrizations. Following the IPCC classification (2006 to 2019), in the study area, (i) the climate fell in a “warm temperate, dry” region (comparing
Table 1 data), (ii) soils were classified as “high-activity clay soils” (
Table 3), (iii) land use was “long-term cultivated” and “cropland remaining as cropland”. However, the SOCS predicted by the IPCC model changed in S0 and S1 due to variations in the soil management. The steady-state (S0) management fell in the “full tillage” class due to annual arable crops with generally full inversions, and organic inputs resulted “medium”, according to the IPCC definition, because the annual crop residues were returned to the field, and N-fixing crop (FB) was present in the cropping system. Conversely, as a result of semi-perennial CAR cultivation (S1), tillage occurred only six times in the 20-years scenario (0.3 frequency), so the cropping system could be attributed to the “no till” IPCC class, and the estimated residues supplied in 20 years increased from 104 Mg ha
−1 in S0 to 229 Mg ha
−1 in S1, taking into account both agricultural cropping systems and the data reported in
Table 3. For that reason, the organic inputs in S1 were attributable to the “high without manure” IPCC class.
The SOMBIT parametrization provided k
1 from the literature, except CAR R
bg, that has been estimated. DW and FB R
ag was 0.10 and 0.08, respectively, whereas R
bg was 0.15 for both crops [
92]. CAR R
ag was 0.18, as experimentally calculated by Razza et al. [
24], whereas R
bg was estimated considering that roots of perennial plants have higher decay resistance than annual ones, due to the higher lignin contents [
93]. In detail, k
1 was estimated as 0.27, increasing the k
1 R
bg value applied for annual crops by the perennial and annual RPM ratio applied in RothC (1.80).
In RothC, the arable crop default value (1.44) of the DPM/RPM ratio was used for FB and DW residues, whereas, for CAR residues, 1 and 0.35 ratios were used for R
ag and R
bg, respectively. The DPM/RPM R
ag value means 50% DPM and 50% RPM and is intermediate between the typical value for arable crops and scrubs (1.44 and 0.67, respectively). The DPM/RPM R
bg value was lower following Kätterer et al. [
93] and was assumed as the ratio of 0.26% DPM and 0.74% RPM.
The introduction of CAR (S1) led to a 75-kg C ha
−1 annual increase applying the IPCC tool (2019) refining default values (
Figure 3). This value seems rather underestimated compared with the observed data reported in
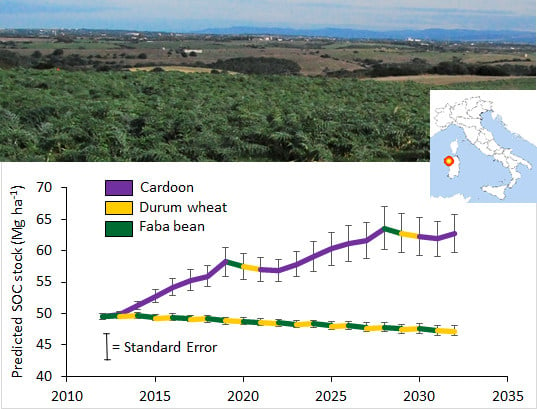
Figure 2. Indeed, for both RMSE and RE statistics (
Table 5), the IPCC tool showed the lowest performance, because it did not accurately predict either the SOC trend or its change. In the SOMBIT and RothC models, the simulated values remained within, or very close to, the standard error of the measured values. The high model performances in simulating the measured data in the studied area gave high reliability to the simulation in the perspective of the next 20 years. However, the IPCC tool results were preserved mainly to compare the predictions obtained with other models. Indeed, the increase in SOCS predicted by the SOMBIT and RothC models resulted higher than the IPCC model (
Figure 3), because they considered even the annual residue quantities supplied and the specific soil features. This improvement in input quantification allowed to assess the different field conditions, reducing the uncertainty due to IPCC wide classes tailored for a national scale.
In S0, SOCS predicted by SOMBIT and RothC decreased, on average, by 267 ± 14 and 61 ± 0.3 kg C ha
−1 per year. These findings are consistent with a decrease observed by Mazzoncini et al. [
94] in a 28-years trial in similar conditions. Contrariwise, in S1, SOCS predicted by SOMBIT and RothC increased in average by 905 ± 110 and 1009 ± 53 kg C ha
−1 per year, respectively. The higher increase occurred in the last year of CAR cultivation for both models when, at explant, all roots were left in the soil.
The long-term prediction highlighted the positive effect of CAR introduction in the rainfed two-year FB-DW rotation in terms of SOC sequestration. In this study, the SR was about 70% greater than that estimated in different cropping systems [
67], resulting from the high C input from plant residues and a simultaneous reduction of C loss by no soil disturbance. Furthermore, the predicted increase is remarkable if related to the carbon footprint of CAR cultivated without organic input in the same area, as estimated by Razza et al. [
24] and Cocco et al. [
95], in 1.7 and 2.1 MgCO
2 eq ha
−1, respectively. Indeed, it is possible to convert the change of the SOCS in potential CO
2 emissions by the ratio of CO
2 and carbon molecular weights: 1 Mg ha
−1 of SOCS corresponds to 3.66 Mg of CO
2 [
96,
97]; hence, the predicted SOCS increase in S1 can probably offset the carbon footprint of CAR. The 4p1000 initiative [
98], despite the criticism on a global-scale application [
99], could be an interesting target at the farm scale, applying the formula suggested by Priori et al. [
97]. It allows to compare the 4‰ target SOCS increase with results of the model predictions. In the investigated fields, to reach the target, the SOCS after 20 years should be 53.9 ± 1.4 Mg C ha
−1, a result widely achieved in S1 following the RothC and SOMBIT models, while not in S0 (
Figure 3).
An explanation to better understand the increase of SOCS, both observed and predicted in 20 years, might be attributed to the reduced bare soil introducing CAR (S1-72% bare soil days than S0), consequently reducing the SOCS mineralization.
Thus, the S1 scenario represents a possible option for farmers to adopt a more sustainable cropping system able to decrease C losses and to increase C inputs in comparison with the traditional systems.