Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting
Abstract
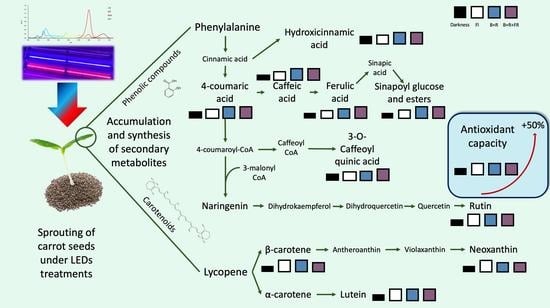
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material and Sprouting Conditions
2.3. Light Treatments Used during the Photoperiod
2.4. Morphological Characteristics
2.5. Extraction and Determination of Total Phenolic Content
2.6. Individual Phenolic Content Analyses
2.7. Total Antioxidant Capacity
2.8. Extraction and Analysis of Carotenoids
2.9. Statistical Analyses
3. Results
3.1. Morphological Characteristics of Carrot Sprouts
3.2. Total Phenolic Content
3.3. Total Antioxidant Capacity
3.4. Individual Phenolic Content
3.5. Carotenoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 11877–11879. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Nat. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Bliznikas, Z.; Žukauskas, A.; Samuoliene, G.; Viršile, A.; Brazaityte, A.; Jankauskiene, J.; Duchovskis, P.; Novičkovas, A. Effect of supplementary pre-harvest LED lighting on the antioxidant and nutritional properties of green vegetables. Acta Hortic. 2012, 939, 85–91. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 88–117. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chory, J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Blankenship, R.E. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011, 16, 426–431. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Kwack, Y.; Kim, K.K.; Hwang, H.; Chun, C. Growth and quality of sprouts of six vegetables cultivated under different light intensity and quality. Hortic. Environ. Biotechnol. 2015, 56, 437–443. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef]
- Seo, J.M.; Arasu, M.V.; Kim, Y.B.; Park, S.U.; Kim, S.J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 117, 204–213. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.S.; Tan, G.F.; Li, T.; Wang, Y.H.; Khadr, A.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- Castillejo, N.; Martínez-Hernández, G.B.; Monaco, K.; Gómez, P.A.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Preservation of bioactive compounds of a green vegetable smoothie using short time-high temperature mild thermal treatment. Food Sci. Technol. Int. 2017, 23, 46–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés–Hernández, F. Postharvest Led Lighting: Effect of Red, Blue, and Far Red on Quality Of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2020, 101, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Parks, B.M.; Hoecker, U.; Spalding, E.P. Light-induced growth promotion by SPA1 counteracts phytochrome-mediated growth inhibition during de-etiolation. Plant Physiol. 2001, 126, 1291–1298. [Google Scholar] [CrossRef] [Green Version]
- Parks, B.M.; Spalding, E.P. Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 14142–14146. [Google Scholar] [CrossRef] [Green Version]
- Folta, K.M.; Spalding, E.P. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001, 26, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kuno, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of irradiation patterns and light quality of red and blue light-emitting diodes on growth of leaf lettuce (Lactuca sativa L.”Greenwave”). Environ. Control Biol. 2017, 55, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of light-emitting diodes on the accumulation of glucosinolates and phenolic compounds in sprouting canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.K.; Chen, Y.Y.; Hu, T.T.; Zhang, S.J.; Zhang, Y.H.; Zhao, T.Y.; Yu, H.E.; Kang, Y.F. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Y.; Duan, F.; Du, X.; Yang, Q.; Zheng, Y. Improvement of the Growth and Nutritional Quality of Two-leaf-color Pak Choi by Supplemental Alternating Red and Blue Light. HortScience 2020, 1, 118–125. [Google Scholar] [CrossRef]
- Hossen, M.Z. Light emitting diodes increase phenolics of buckwheat (Fagopyrum esculentum) sprouts. J. Plant Interact. 2007, 2, 71–78. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, S.Y.; Oh, M.M. Growth and phenolic compounds of Crepidiastrum denticulatum under various blue light intensities with a fixed phytochrome photostationary state using far-red light. Hortic. Environ. Biotechnol. 2019, 60, 199–206. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B Biol. 2019, 193, 18–30. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]





| Days at 20 °C | Treatment | Hypocotyl Length (cm) | Root Length (cm) | Sprout Length (cm) | H/R Ratio |
|---|---|---|---|---|---|
| Growing under darkness conditions | |||||
| 3 dD | Darkness | - | 0.24 ± 0.03 | 0.24 ± 0.03 | - |
| 7 dD | Darkness | 1.10 ± 0.04 | 1.52 ± 0.18 | 2.61 ± 0.16 | 0.83 ± 0.08 |
| + days under a 16 h light/8 h darkness photoperiod | |||||
| 7 dD+3 dP | Darkness | 2.51 ± 0.22 A b | 1.17 ± 0.21 B b | 3.43 ± 0.58 b | 3.51 ± 0.88 A |
| Fl | 1.17 ± 0.09 B | 2.17 ± 0.49 A | 3.34 ± 0.44 | 0.64 ± 0.22 B | |
| B+R | 1.00 ± 0.10 B b | 2.20 ± 0.33 A | 3.01 ± 0.64 | 0.50 ± 0.08 B b | |
| B+R+FR | 1.20 ± 0.14 B b | 1.65 ± 0.24 AB b | 2.85 ± 0.30 b | 0.95 ± 0.27 B | |
| 7 dD+7 dP | Darkness | 4.51 ± 0.77 A a | 2.41 ± 0.32 AB a | 6.92 ± 1.04 A a | 2.64 ± 0.80 A |
| Fl | 1.64 ± 0.56 B | 2.08 ± 0.27 B | 3.72 ± 0.83 B | 1.14 ± 0.31 B | |
| B+R | 1.22 ± 0.10 B b | 2.10 ± 0.25 B | 3.32 ± 0.28 B | 0.63 ± 0.08 BC b | |
| B+R+FR | 1.21 ± 0.07 B b | 3.09 ± 0.43 A a | 4.30 ± 0.50 B a | 0.43 ± 0.00 BC | |
| 7 dD+10 dP | Darkness | 5.68 ± 0.67 A a | 1.23 ± 0.34 b | 6.91 ± 0.68 A a | 4.86 ± 0.63 A |
| Fl | 2.04 ± 0.08 B | 2.31 ± 0.11 | 4.35 ± 0.18 B | 0.89 ± 0.03 B | |
| B+R | 1.86 ± 0.16 B a | 2.38 ± 0.58 | 4.24 ± 0.74 B | 1.07 ± 0.14 B a | |
| B+R+FR | 1.80 ± 0.16 B b | 2.03 ± 0.75 ab | 3.84 ± 0.62 B ab | 1.06 ± 0.44 B | |
| Days at 20 °C | ||||||
|---|---|---|---|---|---|---|
| Treatment | Seed | 3 dD | 7 dD | 7 dD+3 dP | 7 dD+7 dP | 7 dD+10 dP |
| DPPH | ||||||
| Darkness | 834.7 ± 39.6 d | 1508.6 ± 81.0 c | 4351.8 ± 366.6 b | 7579.4 ± 179.8 B a | 4324.5 ± 25.6 B b | 8403.3 ± 888.4 C a |
| Fl | 10916.7 ± 58.9 A a | 11921.9 ± 1278.3 A a | 11803.7 ± 234.5 B a | |||
| B+R | 11352.7 ± 919.3 A b | 13019.6 ± 1065.8 A ab | 13877.1 ± 371.8 A a | |||
| B+R+FR | 11737.9 ± 817.4 A a | 11390.6 ± 1338.7 A a | 12920.9 ± 563.2 AB a | |||
| FRAP | ||||||
| Darkness | 3230.7 ± 194.0 d | 2720.3 ± 261.2 d | 5106.3 ± 377.6 c | 7292.7 ± 357.7 C b | 7149.8 ± 449.5 C b | 8653.2 ± 247.6 C a |
| Fl | 11425.9 ± 451.6 B b | 13421.7 ± 705.6 A a | 10182.2 ± 742.4 C b | |||
| B+R | 12035.0 ± 561.4 AB b | 11005.4 ± 1267.0 B b | 18148.6 ± 563.4 A a | |||
| B+R+FR | 12983.3 ± 449.5 A b | 12050.6 ± 841.9 AB b | 15121.1 ± 683.9 B a | |||
| ABTS | ||||||
| Darkness | 1944.8 ± 144.4 d | 1722.3 ± 158.3 d | 5522 ± 395.4 c | 7944.5 ± 636.3 B b | 6862.6 ± 123.2 B b | 11400.4 ± 1152.5 C a |
| Fl | 13378.2 ± 982.2 A b | 16069.7 ± 1257.9 A a | 13825.3 ± 669.9 B ab | |||
| B+R | 13853.0 ± 796.1 A a | 14766.9 ± 890.4 A a | 15253.6 ± 295.4 A a | |||
| B+R+FR | 13633.0 ± 812.0 A a | 14775.8 ± 1085.1 A a | 15529.4 ± 518.8 A a | |||
| Treatment | Day of Analysis | Neochlorogenic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Hydroxycinnamic Acid | Rutin | 1,2-Disinapoyl-1′-Ferulolylgentiobiose | Total Individual Phenols |
|---|---|---|---|---|---|---|---|---|---|---|
| Seed | 0dD | 131.9 ± 16.6 ab | 7.0 ± 0.17 c | 0.2 ± 0.02 c | 207.8 ± 17.8 c | 0.2 ± 0.0 c | 0.5 ± 0.0 c | 6.2 ± 0.5 c | 0.4 ± 0.0 d | 354.1 ± 33.4 e |
| Growing under darkness conditions | ||||||||||
| 3dD | 99.0 ± 11.7 bc | 8.9 ± 4.6 c | 5.4 ± 2.4 c | 129.5 ± 11.0 d | 12.3 ± 2.8 c | 1.1 ± 0.0 c | 7.7 ± 0.7 c | 4.1 ± 0.7 d | 268.1 ± 34.8 e | |
| 7dD | 127.5 ± 8.6 ab | 420.5 ± 20.9 b | 42.7 ± 8.6 b | 246.5 ± 23.3 bc | 1396.8 ± 45.1 b | 37.5 ± 3.6 c | 141.0 ± 17.3 b | 20.8 ± 6.4 c | 2433.3 ± 110.6 d | |
| + days under a 16h light/8h darkness photoperiod | ||||||||||
| Darkness | 7 dD+3 dP | 68.7 ± 8.2 C c | 506.1 ± 52.6 D b | 77.7 ± 4.7 B a | 282.9 ± 29.1 B b | 1841.7 ± 111.4 D a | 85.9 ± 22.7 B b | 158.3 ± 0.8 C ab | 53.6 ± 7.9 C b | 3074.9 ± 164.4 D c |
| 7 dD +7 dP | 129.2 ± 1.9 C ab | 444.5 ± 31.5 C b | 77.7 ± 20.7 B a | 400.7 ± 40.1 B a | 1972.2 ± 142.4 C a | 358.3 ± 18.0 C a | 126.2 ± 18.4 C b | 38.7 ± 6.8 C b | 3547.5 ± 269.3 C b | |
| 7 dD+10 dP | 134.9 ± 1.5 C a | 943.2 ± 52.0 C a | 102.7 ± 13.9 B a | 418.5 ± 7.1 B a | 1852.5 ± 135.4 C a | 366.1 ± 20.3 A a | 182.5 ± 12.3 B a | 81.8 ± 7.0 C a | 4082.2 ± 151.5 C a | |
| Fl | 7 dD+3 dP | 211.6 ± 1.4 A a | 1800.7 ± 135.3 C a | 212.5 ± 2.9 A a | 470.7 ± 41.3 A | 2654.9 ± 53.5 C b | 149.7 ± 15.2 B b | 909.2 ± 9.3 B a | 158.1 ± 14.2 B b | 6567.5 ± 220.8 C b |
| 7 dD+7 dP | 194.4 ± 5.9 A b | 1886.7 ± 91.3 B a | 191.7 ± 19.1 A a | 526.2 ± 36.6 AB | 3226.6 ± 262.9 B a | 460.1 ± 46.7 B a | 804.9 ± 51.9 A a | 194.2 ± 13.7 B a | 7484.8 ± 303.8 B a | |
| 7 dD+10 dP | 168.1 ± 4.1 B c | 1437.4 ± 131.0 B b | 110.8 ± 3.7 B b | 424.0 ± 45.2 B | 2816.6 ± 172.2 B ab | 398.2 ± 28.0 A a | 506.5 ± 55.5 A b | 157.6 ± 11.8 B b | 6019.3 ± 384.5 B b | |
| B+R | 7 dD+3 dP | 150.7 ± 15.8 B b | 2202.3 ± 31.3 B ab | 197.0 ± 15.4 A a | 524.6 ± 30.1 A | 3968.8 ± 144.1 B | 279.3 ± 35.8 A b | 949.3 ± 63.7 B a | 159.2 ± 6.5 B b | 8431.1 ± 171.1 B |
| 7 dD+7 dP | 170.0 ± 16.2 B b | 1985.1 ± 128.0 B b | 132.1 ± 11.5 AB b | 613.0 ± 54.7 A | 4423.9 ± 327.4 A | 615.0 ± 24.0 A a | 550.2 ± 14.8 B b | 231.9 ± 23.2 AB a | 8721.1 ± 567.7 AB | |
| 7 dD+10 dP | 217.6 ± 17.4 A a | 2489.8 ± 202.5 A a | 206.3 ± 5.8 A a | 602.6 ± 18.4 A | 4205.2 ± 403.3 A | 569.3 ± 16.9 A a | 601.4 ± 58.6 A b | 262.0 ± 15.1 A a | 9154.2 ± 835.5 A | |
| B+R+FR | 7 dD+3 dP | 194.9 ± 22.5 A | 2967.2 ± 126.1 A a | 227.9 ± 17.3 A a | 547.3 ± 69.6 A | 4677.7 ± 281.2 A | 322.4 ± 25.9 A b | 1213.1 ± 69.5 A a | 337.2 ± 29.2 A a | 10487.7 ± 390.7 A |
| 7 dD+7 dP | 206.8 ± 1.8 A | 2601.3 ± 155.6 A b | 162.6 ± 9.0 A b | 618.7 ± 71.9 A | 4385.5 ± 440.1 A | 537.3 ± 9.4 AB a | 616.5 ± 12.7 B b | 272.9 ± 18.1 A b | 9401.7 ± 648.1 A | |
| 7 dD +10 dP | 201.2 ± 12.5 A | 2492.4 ± 89.3 A b | 220.4 ± 8.6 A a | 657.9 ± 38.0 A | 4631.0 ± 164.4 A | 512.0 ± 10.7 A a | 632.9 ± 23.9 A b | 296.7 ± 20.7 A ab | 9644.4 ± 114.2 A | |
| Treatment | Day of Analysis | All-Trans Neoxanthin | 13-cis or 13′-cis Lutein | All-Trans Lutein | 9-cis or 9′-cis Lutein | 15-cis β-Carotene | All-trans β-Carotene | 9-cis β-Carotene | Total Carotenoids |
|---|---|---|---|---|---|---|---|---|---|
| Seed | 0 dD | 0.3 ± 0.0 d | 2.4 ± 0.1 | 2.0 ± 0.1 d | 0.00 ± 0.00 c | 2.4 ± 0.1 c | 0.3 ± 0.0 c | 0.0 ± 0.0 c | 7.3 ± 0.2 d |
| Growing under darkness conditions | |||||||||
| 3 dD | 0.7 ± 0.1 cd | 2.5 ± 0.1 | 2.0 ± 0.4 d | 0.00 ± 0.00 c | 2.3 ± 0.1 c | 0.3 ± 0.1 c | 0.0 ± 0.0 c | 7.8 ± 0.8 d | |
| 7 dD | 1.6 ± 0.1 b | 2.5 ± 0.2 | 12.1 ± 2.1 c | 0.18 ± 0.05 b | 11.1 ± 1.8 c | 1.4 ± 0.2 c | 2.0 ± 0.4 c | 30.8 ± 4.7 c | |
| + days under 16h light/8h darkness photoperiod | |||||||||
| Darkness | 7 dD+3 dP | 2.0 ± 0.3 C ab | 2.4 ± 0.3 C | 15.6 ± 0.3 B c | 0.39 ± 0.02 B b | 25.8 ± 9.9 D b | 7.8 ± 1.2 D b | 14.7 ± 1.9 C b | 68.6 ± 11.7 D b |
| 7 dD+7 dP | 1.3 ± 0.1 C bc | 2.2 ± 0.1 C | 22.0 ± 0.1 C b | 0.23 ± 0.03 C a | 31.9 ± 7.6 D ab | 7.7 ± 0.7 C b | 13.0 ± 1.8 C b | 78.4 ± 9.8 D b | |
| 7 dD+10 dP | 2.5 ± 0.6 C a | 2.5 ± 0.1 B | 37.6 ± 4.0 B a | 0.24 ± 0.02 B b | 41.1 ± 1.5 D a | 12.2 ± 0.9 C a | 18.2 ± 1.4 B a | 114.3 ± 7.9 C a | |
| Fl | 7 dD+3 dP | 8.1 ± 0.7 A c | 9.4 ± 0.4 A c | 53.0 ± 7.6 A b | 0.54 ± 0.05 A | 118.7 ± 1.6 B b | 17.7 ± 1.1 B b | 23.6 ± 1.2 B b | 231.2 ± 11.0 B b |
| 7 dD+7 dP | 15.8 ± 1.2 A b | 18.5 ± 2.2 A b | 119.0 ± 6.0 A a | 0.46 ± 0.03 B | 138.5 ± 5.5 A a | 35.6 ± 3.0 A a | 41.8 ± 2.2 A a | 369.6 ± 17.8 A a | |
| 7 dD+10 dP | 20.9 ± 0.3 A a | 26.0 ± 0.7 A a | 132.5 ± 5.3 A a | 0.50 ± 0.01 A | 134.7 ± 6.6 B a | 36.7 ± 2.4 AB a | 46.0 ± 2.9 A a | 397.2 ± 12.4 A a | |
| B+R | 7 dD+3 dP | 5.1 ± 1.0 B c | 6.3 ± 1.3 B c | 55.6 ± 5.0 A c | 0.49 ± 0.02 A b | 139.7 ± 1.9 A a | 26.7 ± 0.7 A b | 33.9 ± 1.7 A b | 267.8 ± 11.6 A b |
| 7 dD+7 dP | 11.6 ± 1.5 B b | 14.3 ± 0.9 B b | 93.7 ± 9.8 B b | 0.43 ± 0.02 B b | 110.7 ± 10.2 B b | 30.0 ± 3.1 AB b | 36.2 ± 2.8 B b | 297.0 ± 27.0 B b | |
| 7 dD+10 dP | 17.6 ± 1.1 B a | 23.8 ± 1.6 A a | 127.3 ± 12.0 A a | 0.56 ± 0.03 A a | 149.4 ± 5.0 A a | 42.7 ± 1.7 A a | 47.1 ± 3.1 A a | 408.5 ± 13.5 A a | |
| B+R+FR | 7 dD+3 dP | 5.1 ± 0.8 B c | 6.4 ± 1.8 B c | 50.5 ± 9.7 A c | 0.46 ± 0.05 AB b | 78.7 ± 1.6 C | 13.4 ± 1.3 C b | 19.2 ± 3.0 BC b | 173.9 ± 13.2 C c |
| 7 dD+7 dP | 11.9 ± 0.6 B b | 16.2 ± 1.5 AB b | 83.3 ± 8.6 B b | 0.55 ± 0.02 A a | 80.4 ± 7.4 C | 27.8 ± 2.3 B a | 30.8 ± 1.2 B a | 251.0 ± 5.9 C b | |
| 7 dD+10 dP | 18.9 ± 0.9 AB a | 25.2 ± 1.2 A a | 120.7 ± 9.2 A a | 0.53 ± 0.02 A ab | 86.0 ± 4.5 C | 32.9 ± 3.4 B a | 41.3 ± 2.8 A a | 325.5 ± 13.7 B a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11020304
Martínez-Zamora L, Castillejo N, Gómez PA, Artés-Hernández F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy. 2021; 11(2):304. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11020304
Chicago/Turabian StyleMartínez-Zamora, Lorena, Noelia Castillejo, Perla A. Gómez, and Francisco Artés-Hernández. 2021. "Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting" Agronomy 11, no. 2: 304. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11020304