Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium japonicum for Soybean (Glycine max (L.) Merr.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Characterization, and Identification of B. japonicum
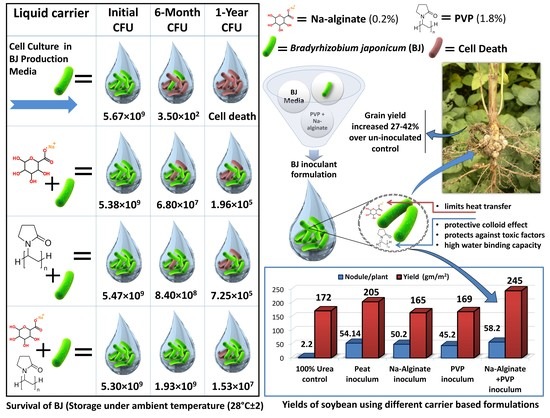
2.2. Development of Liquid Inoculant
2.2.1. Microbial Production Media and Cell Culture in Fermenter
2.2.2. Liquid Inoculant Preparation
2.3. Field Evaluation
2.3.1. Field Trial with Different Carrier Based Inoculation
2.3.2. Field Trial with Different Nitrogen Doses
2.4. Statistical Analysis
3. Results
3.1. Identification of the B. japonicum (APEXBJ2) Strain
3.2. Survival of B. japonicum (APEXBJ2) Strain in Liquid Formulation
3.3. Field Experiments
3.3.1. Evaluation of Carrier-Based Formulations
3.3.2. Evaluation of Liquid Formulation with Different Nitrogen Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PVP | Polyvinylpyrrolidone |
| PEG | Polyethylene glycol |
| HDPE | High density polyethylene |
| BARI | Bangladesh Agriculture Research Institute |
| BINA | Bangladesh Institute of Nuclear Agriculture |
| B. japonicum | Bradyrhizobium japonicum |
| NCBI | National Center for Biotechnology Information |
| USA | United States of America |
| TSP | Triple super phosphate |
| MOP | Muriate of potash |
References
- Wilcox, J. World distribution and trade of soybean. In Soybean: Improvement, Production, and Uses; Boerma, H.R., Specht, J.E., Eds.; American Society of Agronomy: Madison, WI, USA, 2004; pp. 1–14. [Google Scholar]
- Vieira, R.F.; Mendes, I.C.; Reis-Junior, F.B.; Hungria, M. Symbiotic nitrogen fixation in tropical food grain legumes: Current status. In Microbes for Legume Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Vienna, Austria, 2010; pp. 427–472. [Google Scholar]
- Youseif, S.H.; El-Megeed, F.H.A.; Saleh, S.A. Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low-fertility soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. Comparing symbiotic performance and physiological responses of two soybean cultivars to arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2016, 26, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Navarro, D.N.; Margaret Oliver, I.; Albareda Contreras, M.; Ruiz-Sainz, J.E. Soybean interactions with soil microbes, agronomical and molecular aspects. Agron. Sustain. Dev. 2011, 31, 173–190. [Google Scholar] [CrossRef] [Green Version]
- Madrzak, C.J.; Golinska, B.; Kroliczak, J.; Pudelko, K.; Lazewska, D.; Lampka, B.; Sadowsky, M.J. Diversity among field populations of Bradyrhizobium japonicum in Poland. Appl. Environ. Microbiol. 1995, 61, 1194–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; Abd_Allah, E.F. Impact of soil salinity on the plant-growth–promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 2017, 24, 1601–1608. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Ma, H.; Alimov, J.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Response of Soybean to hydrochar-based Rhizobium inoculation in loamy sandy soil. Microorganisms 2020, 8, 1674. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A.; Palni, L.M. Carrier-based preparations of plant growth-promoting bacterial inoculants suitable for use in cooler regions. World J. Microb. Biotechnol. 2005, 21, 941–945. [Google Scholar] [CrossRef]
- Abd El-Fattah, D.A.; Eweda, W.E.; Zayed, M.S.; Hassanein, M.K. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann. Agric. Sci. 2013, 58, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Htwe, A.Z.; Moh, S.M.; Soe, K.M.; Moe, K.; Yamakawa, T. Effect of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea, and soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Ardakani, S.S.; Hedari, A.; Tayebi, L.; Mohammadi, M. Promotion of cotton seedlings growth characteristics by development and use of new bioformulations. Int. J. Bot. 2010, 6, 95–100. [Google Scholar] [CrossRef]
- Berninger, T.; Lopez, O.G.; Bejarano, A.; Preininger, C. Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant growth promoting rhizobacteria: Constraints in bioformulation, commercialization, and future strategies. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 18, pp. 97–116. [Google Scholar]
- Tittabutr, P.; Payakapong, W.; Teaumroong, N.; Singleton, P.W.; Boonkerda, N. Growth, survival and field performance of Bradyrhizobial liquid inoculant formulations with polymeric additives. Sci. Asia 2007, 33, 69–77. [Google Scholar] [CrossRef]
- Girisha, H.C.; Brahmaprakash, G.P.; Mallesha, B.C. Effect of osmoprotectant (PVP-40) on survival of Rhizobium in different inoculants formulation and nitrogen fixation in cowpea. Geobios-Jodhpur- 2006, 33, 151–156. [Google Scholar]
- Sukhovitskaia, L.A.; Safronova, G.V.; Klyshko, G.M.; Korolenok, N.V. Survival of Rhizobium in monoculture and binary population with rhizosphere bacteria. Prikl. Biokhim. Mikrobiol. 2002, 38, 73–78. (In Russian) [Google Scholar] [PubMed]
- Hubálek, Z. Protectant used in the cryopreservation of microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- Ondieki, D.K.; Nyaboga, E.N.; Wagacha, J.M.; Mwaura, F.B. Morphological and genetic diversity of Rhizobian nodulating cowpea (Vigna unguiculata L.) from agricultural soils of lower eastern Kenya. Int. J. Microbiol. 2017, 2017, 8684921. [Google Scholar] [CrossRef] [Green Version]
- Somasegaran, P.H.; Hoben, H. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer: Berlin/Heidelberg, Germany, 1994; Volume Xvi, p. 450. [Google Scholar]
- Hoagland, D.R. Optimum nutrient solutions for plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA 6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, A.; Suneja, S.; Yadav, A.; Anand, R.C. Influence of different additives on shelf life of Rhizobial inoculants for mungbean (Vigna radiata L.). Int. J. Recent Sci. Res. 2015, 6, 4338–4342. [Google Scholar]
- Roughley, R.J. The preparation and use of legume seed inoculants. Plant Soil 1970, 32, 675–701. [Google Scholar] [CrossRef]
- FRG. Fertilizer Recommendation Guide; Bangladesh Agriculture Research Council (BARC): Farmgate, Dhaka, 2012; 274p.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://www.R-project.org (accessed on 14 April 2021).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PCMR). R Package Version 4.1. 2014. Available online: http://cran.r-project/package=PCMR (accessed on 14 April 2021).
- Roughley, R.J.; Vincent, J.M. Growth and survival of Rhizobium spp. in peat culture. J. Appl. Bacteriol 1967, 30, 362–376. [Google Scholar] [CrossRef]
- Taurian, T.; Anzuay, M.S.; Angelini, J.G.; Tonelli, M.L.; Ludueña, L.; Pena, D.; Ibáñez, F.; Fabraet, A. Phos-phate-solubilizing peanut associated bacteria: Screening for plant growth promoting activities. Plant Soil 2010, 329, 421–431. [Google Scholar] [CrossRef]
- Amalraj, E.L.D.; Venkateswarlu, B.; Desai, S.; Kumar, G.P.; Ahmed, S.K.M.H.; Meenakshi, T.; Sultana, U.; Pinisetty, S.; Man-gamoori, L.N. Effect of polymeric additives, adjuvants, surfactants on survival, stability and plant growth promoting ability of liquid bioinoculants. J. Plant. Physiol. Pathol. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Schulz, T.J.; Thelen, K.D. Soybean seed inoculant and fungicidal seed treatment effects on soybean. Crop Sci. 2008, 48, 1975–1983. [Google Scholar] [CrossRef]
- Singleton, P.; Keyser, H.; Sande, E. Development and evaluation of liquid Inoculants. In Inoculants and Nitrogen Fixation of Legumes in Vietnam; Herridge, D., Ed.; ACIAR: Canberra, Austrilia, 2002; Volume 109, pp. 52–66. [Google Scholar]
- Viveganandan, G.; Jauhri, K.S. Growth and survival of phosphate-solubilizing bacteria in calcium alginate. Microbiol. Res. 2000, 155, 205–207. [Google Scholar] [CrossRef]
- Biradar, B.J.P.; Santhosh, G.P. Role of polymeric additives in formulation, shelf-life and bioefficacy of liquid inoculant of Pseudomonas fluoresens. Int. J. Pure Appl. Biosci. 2018, 6, 123–133. [Google Scholar] [CrossRef]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume seed inoculation technology—A review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Zohar-Perez, C.; Ritte, E.; Chernin, L.; Chet, I.; Nussinovitch, A. Preservation of chitinolytic Pantoae agglomerans in a viable form by cellular dried alginate-based carriers. Biotechnol. Prog. 2002, 18, 1133–1140. [Google Scholar] [CrossRef]
- Manikandan, R.; Saravanakumar, D.; Rajendran, L.; Raguchander, T.; Samiyappan, R. Standardization of liquid formulation of Pseudomonas fluorescens Pf1 for its efficacy against Fusarium wilt of tomato. Biol. Control 2010, 54, 83–89. [Google Scholar] [CrossRef]
- Thao, T.Y.; Singleton, P.W.; Herridge, D. Inoculation responses of soybean and liquid inoculants as an alternative to peat-based inoculants. In Inoculants and Nitrogenffixation of Legumes in Vietnam; Herridge, D., Ed.; ACIAR: Canberra, Austrilia, 2002; Volume 109, pp. 67–74. [Google Scholar]
- Albareda, M.; Rodriguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculant: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Rice, W.A.; Clayton, G.W.; Olsen, P.E.; Lupwayi, N.Z. Rhizobial inoculant formulations and soil pH influence pea nodulation and nitrogen fixation. Can. J. Soil Sci. 2000, 80, 395–400. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Slinkard, A.E.; Walley, F.L. Evaluation of rhizobial inoculation methods for chickpea. Agron. J. 2002, 94, 851–859. [Google Scholar] [CrossRef]
- Clayton, G.W.; Rice, W.A.; Lupwayi, N.Z.; Johnston, A.M.; Lafond, G.P.; Grant, C.A.; Walley, F. Inoculant formula-tion and fertilizer nitrogen effects on field pea: Nodulation, N2 fixation and nitrogen partitioning. Can. J. Plant Sci. 2004, 84, 79–88. [Google Scholar] [CrossRef]
- Clayton, G.W.; Rice, W.A.; Lupwayi, N.Z.; Johnston, A.M.; Lafond, G.P.; Grant, C.A.; Walley, F. Inoculant formulation and fertilizer nitrogen effects on field pea: Crop yield and seed quality. Can. J. Plant Sci. 2004, 84, 89–96. [Google Scholar] [CrossRef]
- Egamberdiyeva, D.; Qarshieva, D.; Davranov, K. The use of Bradyrhizobium to enhance growth and yield of soybean in calcareous soil in Uzbekistan. J. Plant Growth Regul. 2004, 1, 54–57. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-i.; Teaumroong, N. Co-Inoculation of Bacillus velezensis Strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]






| Liquid Carrier | Colony Forming Units (CFU)/mL | |||||
|---|---|---|---|---|---|---|
| Initial CFU | 120 Days | 180 Days | 240 Days | 300 Days | 360 Days | |
| Control | 5.67 × 109 | 4.53 × 105 | 3.50 × 102 | - | - | - |
| Na-Alginate (0.2%) + PVP (1.8%) | 5.30 × 109 | 2.53 × 109 | 1.93 × 109 | 6.10 × 108 | 5.10 × 107 | 1.53 × 107 |
| PVP (1.8%) | 5.47 × 109 | 4.91 × 109 | 8.40 × 108 | 1.63 × 107 | 1.44 × 106 | 7.25 × 105 |
| Na-Alginate (0.2%) | 5.38 × 109 | 3.70 × 108 | 6.80 × 107 | 4.60 × 106 | 3.90 × 105 | 1.96 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitra, P.; Al-Rashid, J.; Mandal, D.; Azam, M.S.; Rasul, N.M. Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium japonicum for Soybean (Glycine max (L.) Merr.). Agronomy 2021, 11, 1009. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11051009
Maitra P, Al-Rashid J, Mandal D, Azam MS, Rasul NM. Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium japonicum for Soybean (Glycine max (L.) Merr.). Agronomy. 2021; 11(5):1009. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11051009
Chicago/Turabian StyleMaitra, Pulak, Jubair Al-Rashid, Dipa Mandal, Md. Shofiul Azam, and Noorain Munim Rasul. 2021. "Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium japonicum for Soybean (Glycine max (L.) Merr.)" Agronomy 11, no. 5: 1009. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11051009