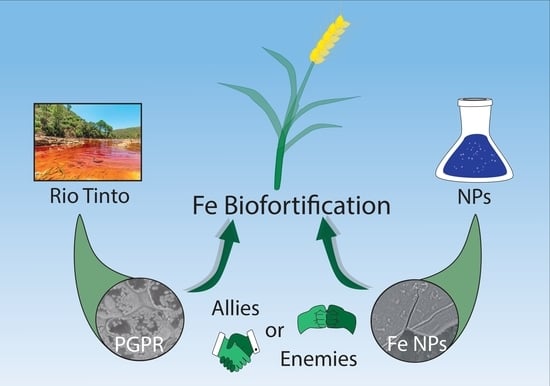
Assessing the Biofortification of Wheat Plants by Combining a Plant Growth-Promoting Rhizobacterium (PGPR) and Polymeric Fe-Nanoparticles: Allies or Enemies?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Tannic Acid-Polyvinyl Alcohol Nanoparticles and Iron Nanoparticles (FeNPs)
2.2. Bacterial Growth Conditions
2.3. Determination of Optimal FeNPs Doses
2.4. Effect of Treatment on Seed Germination, Seedlings Survival, and Root Colonization by Bacillus Aryabhattai RSO25
2.5. Effect of the Treatments on Plant Growth, Physiology, and Iron Accumulation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of FeNPs on the Growth of the Bacterial Strain
3.2. Effect of Treatment on the Germination Rate, Initial Plant Survival, and Root Colonization
3.3. Effect of Treatment on the Growth and Physiology of Wheat Plants
3.4. Effect of the Experimental Treatments on the Concentration of Mineral Nutrients in Plant Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, N. The global challenge of hidden hunger: Perspectives from the field. Proc. Nut. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef]
- Biesalki, H.K. Hidden hunger in the Developed World. In RTGN; Chapter 3; p. 40–50. Available online: https://www.nutri-facts.org/content/dam/nutrifacts/media/media-books/RTGN_chapter_03.pdf (accessed on 25 October 2021).
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC3999603 (accessed on 25 October 2021).
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trends and determinants. Glob. Food Secur. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y.; Wu, Z.; Lv, T. A Bibliometric Analysis on Land Degradation: Current Status, Development, and Future Directions. Land 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sect. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.R.; Venegas, V.; Oliva, J.M.; Sayagués, M.J.; de Miguel, M.; Sánchez-Alcázar, J.A.; Arévalo-Rodríguez, M.; Zaderenko, A.P. Targeted multifunctional tannic acid nanoparticles. RSC Adv. 2016, 6, 7279–7287. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Lurthy, T.; Pivato, B.; Lemanceau, P.; Mazurier, S. Importance of the Rhizosphere Microbiota in Iron Biofortification of Plants. Front. Plant. Sci. 2021, 12, 744445. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of Chitosan-PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [Green Version]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Fakharzadeh, S.; Hafizi, M.; Baghaei, M.; Etesami, M.; Khayamzadeh, M.; Kalanaky, S.; Akbari, M.; Nazaran, M. Using Nanochelating Technology for Biofortification and Yield Increase in Rice. Sci. Rep. 2020, 10, 4351. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant. Growth Reg. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Krzyzowska, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bien, K.; Orlowski, P.; Celichowski, G.; Grobelny, J. Tannic acid modification of metal nanoparticles: Possibility for new antiviral applications. In Micro and Nano Technologies, Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 12; pp. 335–363. [Google Scholar] [CrossRef]
- Saowalak, K.; Titipun, T.; Somchai, T.; Chalermchai, P. Iron(III)-Tannic Molecular Nanoparticles Enhance Autophagy effect and T1 MRI Contrast in Liver Cell Lines. Sci. Rep. 2018, 8, 6647. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T. Reviewing the Tannic Acid Mediated Synthesis of Metal Nanoparticles. J. Nanotechnol. 2014, 954206. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe(III) and Fe(II). J. Anal. Methods Chem. 2019, 3894571. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; de Hollander, M.; Raaijmakers, J.M. The wild side of plant microbiomes. Microbiome 2018, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Curie, C.; Briat, J.F. Iron Transport and Signaling in Plants. Ann. Rev. Plant. Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef]
- Hider, R.C.; Yoshimura, E.; Khodr, H.; von Wirén, N. Competition or complementation: The iron-chelating abilities of nicotianamine and phytosiderophores. New Phytol. 2004, 164, 201–204. [Google Scholar] [CrossRef]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yashveer, S.; Singh, V.; Kaswan, V.; Kaushik, A.; Tokas, J. Green biotechnology, nanotechnology and biofortification: Perspectives on novel environment-friendly crop improvement strategies. Biotechnol. Genet. Eng. Rev. 2014, 30, 113–126. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant. Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 3, 197. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Kloepper, J.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Lee, T.S.; Kolthoff, I.M.; Leussing, D.L. Reaction of Ferrous and Ferric Ions with 1,10-Phenanthroline. II. Kinetics of Formation and Dissociation of Ferrous Phenanthroline. J. Am. Chem. Soc. 1948, 70, 3596–3600. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Caviedes, M.A.; Doukkali, B.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E. Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: Comparison of gram-positive and gram-negative strains. Environ. Sci. Pollut. Res. 2016, 23, 19825–19837. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Mateos-Naranjo, E.; Doukkali, B.; Caviedes, M.A.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Pajuelo, E. Modulation of Spartina densiflora plant growth and metal accumulation upon selective inoculation treatments: A comparison of gram negative and gram positive rhizobacteria. Mar. Pollut. Bull. 2017, 125, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Lv, H.; Zhou, W.; Zhang, Q. Beneficial Effects of Tannic Acid on the Quality of Bacterial Communities Present in High-Moisture Mulberry Leaf and Stylo Silage. Front. Microbiol. 2020, 11, 2754. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2020.586412 (accessed on 25 October 2021). [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; CLSI Document M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Hoagland, D.R.; Arnon, I. The water-culture method for growing plants without soil. In California Agricultural Experiment Station Circular; University of California, College of Agriculture, Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer Study Edition; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Environmental Effects on the Relationship Between the Quantum Yields of Carbon Assimilation and in vivo PsII Electron Transport in Maize. Func. Plant. Biol. 1991, 18, 267–278. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Elarabi, N.I.; Abdelhadi, A.A.; Ahmed, R.H.; Saleh, I.; Arif, I.A.; Osman, G.; Ahmed, D.S. Bacillus aryabhattai FACU: A promising bacterial strain capable of manipulate the glyphosate herbicide residues. Saudi J. Biol. Sci. 2020, 27, 2207–2214. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Alam, J.; Sultana, F.; Iqbal, T. Potential of Iron Nanoparticles to Increase Germination and Growth of Wheat Seedling. J. Nanosci. Adv. Tech. 2015, 1, 14–20. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Pajuelo, E.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bacterial inoculants for enhanced seed germination of Spartina densiflora: Implications for restoration of metal polluted areas. Mar. Pollut. Bull. 2016, 110, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, R.; Zhang, H.; Gehong, W.; Zhefei, L. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020, 20, 38. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, C.; Bakshi, U.; Mallick, I.; Mukherji, S.; Bera, B.; Ghosh, A. Genome-Guided Insights into the Plant Growth Promotion Capabilities of the Physiologically Versatile Bacillus aryabhattai Strain AB211. Front. Microbiol. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Páliz, K.; Rodríguez-Vázquez, R.; Duarte, B.; Caviedes, M.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Caçador, M.I.; Rodríguez-Llorente, I.D.; Pajuelo, E. Investigating the mechanisms underlying phytoprotection by plant growth-promoting rhizobacteria in Spartina densiflora under metal stress. Plant. Biol. 2018, 20, 497–506. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef]
- Alomari, D.Z.; Eggert, K.; von Wirén, N.; Alqudah, A.M.; Polley, A.; Plieske, J.; Ganal, M.W.; Pillen, K.; Röder, M.S. Identifying Candidate Genes for Enhancing Grain Zn Concentration in Wheat. Front. Plant. Sci. 2018, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Das, C.K.; Das, A.; Singh, S.K.; Roy, M.; Kim, H.; Sethy, N.; Kumar, A.; Sharma, R.K.; Singh, S.K.; et al. Seed treatment with iron pyrite (FeS2) nanoparticles increases the production of spinach. RSC Adv. 2014, 4, 58495–58504. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Barroso, D.; Komarek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut. Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef]
- Riaz, A.; Huda, N.; Abbas, A.; Raza, S. Biofortification of Wheat with Iron. Int. J. Adv. Sci. Res. 2017, 3, 69–76. Available online: https://ssjournals.com/index.php/ijasr/article/view/4275 (accessed on 25 October 2021). [CrossRef] [Green Version]
- Aciksoz, S.B.; Yarici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant. Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Siva, G.V.; Benita, L.F.J. Iron Oxide Nanoparticles Promotes Agronomic Traits of Ginger (Zingiber officinale Rosc.). Int. J. Adv. Res. Biol. Sci. 2016, 3, 230–237. [Google Scholar]
- Gomaa, M.A.; Kandil, E.E.; Abdelsalam, N.R.; Al-Jaddadi, M.A.M. Growth, productivity of some rice cultivars in relation to nano-zinc and iron fertilizer. Middle East. J. Agric. Res. 2018, 7, 1352–1358. Available online: http://www.curresweb.com/mejar/mejar/2018/1352-1358.pdf (accessed on 25 October 2021).
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef] [Green Version]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; Watt, M. Microbiome and Exudates of the Root and Rhizosphere of Brachypodium distachyon, a Model for Wheat. PLoS ONE 2016, 11, e0164533. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z. Genotypic differences in micronutrient use efficiency in crops. Commun. Soil Sci. Plant. Anal. 2001, 32, 1163–1186. [Google Scholar] [CrossRef]
- Sagbas, S.; Aktas, N.; Sahiner, N. Modified biofunctional p-(tannic acid) microgels and their antimicrobial activity. Appl. Surf. Sci. Part B 2015, 354, 306–313. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Kibbey, T.C.G.; Strevett, K.A. The effect of nanoparticles on soil and rhizosphere bacteria and plant growth in lettuce seedlings. Chemosphere 2019, 221, 703–707. [Google Scholar] [CrossRef]
- Tian, H.; Kah, M.; Kariman, K. Are Nanoparticles a Threat to Mycorrhizal and Rhizobial Symbioses? A Critical Review. Front. Microbiol. 2019, 10, 1660. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2019.01660 (accessed on 25 October 2021). [CrossRef] [Green Version]
- Sillen, W.M.A.; Thijs, S.; Abbamondi, G.R.; De La Torre Roche, R.; Weyens, N.; White, J.C.; Vangronsveld, C. Nanoparticle treatment of maize analyzed through the metatranscriptome: Compromised nitrogen cycling, possible phytopathogen selection, and plant hormesis. Microbiome 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed]






| Treatments | Number of Plants | Watering Conditions |
|---|---|---|
| Control | 4 pots × 3 plants per pot = 12 plants | Once a week with 1 L of Hoagland solution |
| FeNPs | 4 pots × 3 plants per pot = 12 plants | Once a week with 1 L of Hoagland solution containing 1% (v:v) FeNPs |
| RSO25 | 4 pots × 3 plants per pot = 12 plants | Once a week with 1 L of Hoagland solution. Once a month with 1 L of Hoagland solution and 5 mL of the bacterial culture |
| TT Combined | 4 pots × 3 plants per pot = 12 plants | Once a week with 1 L of Hoagland solution containing 1% (v/v) FeNPs. Once a month with 1 L of Hoagland solution with 1% (v/v) FeNPs and 5 mL of the bacterial culture |
| Parameters determined | ||
| After 7 days | After 30 days | At the end of the experiment (45 days) |
| Bacterial survival and root colonization | Fluorescence of the chlorophyll | Root and Shoot length and biomass (dry weight) |
| Leaf gas exchange (IRGA) | Content in macro and micronutrients | |
| Number, weight, and Fe content of Spikes | ||
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | FeNps | RSO25 | TT Combined |
| Net Photosynthetic rate AN (µmol m−2 s−1) | 12.4 ± 1.5 | 13.4 ± 0.9 | 15.0 ± 2.5 | 11.3 ± 2.0 |
| Stomatal conductance gs (mmol m−2 s−1) | 203.5± 30.2 (a) | 142.0 ± 14.3 (b) | 160.0 ± 45.2 (b) | 155.0 ± 11.5 (b) |
| Intercellular CO2 concentration Ci (µmol mol−1) | 290.0 ± 14.0 (a) | 234.7 ± 9.1 (b) | 237.3 ± 15.9 (b) | 227.0 ± 8.2 (b) |
| Apparent carboxylation efficiency Ce (mmol mol−1) | 0.044 ± 0.001 (a) | 0.057± 0.001(b) | 0.060 ± 0.001(b) | 0.051 ± 0.001 (c) |
| Water use efficiency iWUE (µmol mol−1) | 64.2 ± 9.7 (a) | 94.1 ± 5.9 (b) | 93.0 ± 10.5 (b) | 72.9 ± 4.5 (a) |
| Maximum quantum efficiency of photosystem II (Fv/Fm) | 0.80 ± 0.02 | 0.82 ± 0.01 | 0.81 ± 0.01 | 0.82 ± 0.0 |
| Quantum efficiency of photosystem II (ΦPSII) | 0.20 ± 0.02 (a) | 0.26 ± 0.02 (b) | 0.25 ± 0.01 (b) | 0.20 ± 0.01 (a) |
| Electron transport rate (ETR) | 89.0 ± 8.4 (a) | 107.0 ± 10.0 (b) | 106.1 ± 4.8 (b) | 86.2 ± 7.7 (a) |
| Plant Tissue | Treatment | P (mg.kg−1) | S (mg.kg−1) | Na (mg.kg−1) | K (mg.kg−1) | Ca (mg.kg−1) | Mg (mg.kg−1) | Zn (mg.kg−1) |
|---|---|---|---|---|---|---|---|---|
| Stems | Control | 3302 ± 231 (a) | 3701 ± 230 (a) | 288 ± 34 (a) | 33949 ± 2989 | 2061 ± 234 (a) | 595 ± 78 | 9.79 ± 1.22 (a) |
| FeNPs | 2808 ± 134 (b) | 2972 ± 178 (b) | 168 ± 21 (b) | 33788 ± 2689 | 1803 ± 224 (b) | 574 ± 36 | 8.83 ± 1.65 (a) | |
| RSO25 | 3540 ± 158 (a) | 3869 ± 290 (a) | 155 ± 22 (b) | 32123 ± 4013 | 1773 ± 202 (a) | 575 ± 47 | 6.71 ± 0.76 (b) | |
| TT combined | 2356 ± 104 (c) | 2769 ± 134 (b) | 158 ± 30 (b) | 32105 ± 2.55 | 1492 ± 243 (b) | 559 ± 36 | 8.34 ± 0.56 (a) | |
| Spikes | Control | 4320 ± 341 | 2473 ± 90 | 83 ± 12 (a) | 18873 ± 2501 | 911 ± 77 (a) | 641 ± 55 | 13.91 ± 1.22 |
| FeNPs | 4477 ± 109 | 2420 ± 277 | 73 ± 11 (a) | 17849 ± 1446 | 1236 ± 112 (b) | 627 ± 68 | 15.13 ± 3.04 | |
| RSO25 | 4327 ± 212 | 2560 ± 267 | 70 ± 11 (b) | 16795 ± 1888 | 1055 ± 167 (a) | 632 ± 51 | 11.49 ± 2.64 | |
| TT combined | 4512 ± 255 | 2419 ± 190 | 73 ± 8 (a) | 16807 ± 989 | 1255 ± 131 (b) | 664 ± 43 | 11.76 ± 2.47 | |
| Roots | Control | 1174 ± 144 (a) | 1906 ± 247 (a) | 3330 ± 69 (a) | 12508 ± 1329 (a) | 5503 ± 578 (a) | 587 ± 32 (a) | 32.85 ± 2.25 (a) |
| FeNPs | 982 ± 66 (b) | 1479 ± 117 (b) | 2195 ± 245 (b | 12157 ± 1534 (a) | 5354 ± 626 (a) | 585 ± 44 (a) | 38.56 ± 3.08 (b) | |
| RSO25 | 751 ± 223 (c) | 1231 ± 123 (c) | 2276 ± 201 (b) | 7652 ± 898 (b) | 5060 ± 249 (a) | 475 ± 45 (b) | 41.68 ± 4.29 (c) | |
| TT combined | 1196 ± 172 (a) | 1957 ± 154 (a) | 2825 ± 200 (c) | 11814 ± 1005 (a) | 10489 ± 2688 (b) | 542 ± 32 (a) | 37.28 ± 3.66 (b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merinero, M.; Alcudia, A.; Begines, B.; Martínez, G.; Martín-Valero, M.J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Navarro-Torre, S.; Torres, Y.; et al. Assessing the Biofortification of Wheat Plants by Combining a Plant Growth-Promoting Rhizobacterium (PGPR) and Polymeric Fe-Nanoparticles: Allies or Enemies? Agronomy 2022, 12, 228. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12010228
Merinero M, Alcudia A, Begines B, Martínez G, Martín-Valero MJ, Pérez-Romero JA, Mateos-Naranjo E, Redondo-Gómez S, Navarro-Torre S, Torres Y, et al. Assessing the Biofortification of Wheat Plants by Combining a Plant Growth-Promoting Rhizobacterium (PGPR) and Polymeric Fe-Nanoparticles: Allies or Enemies? Agronomy. 2022; 12(1):228. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12010228
Chicago/Turabian StyleMerinero, Manuel, Ana Alcudia, Belén Begines, Guillermo Martínez, María Jesús Martín-Valero, Jesús Alberto Pérez-Romero, Enrique Mateos-Naranjo, Susana Redondo-Gómez, Salvadora Navarro-Torre, Yadir Torres, and et al. 2022. "Assessing the Biofortification of Wheat Plants by Combining a Plant Growth-Promoting Rhizobacterium (PGPR) and Polymeric Fe-Nanoparticles: Allies or Enemies?" Agronomy 12, no. 1: 228. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12010228