Improving Resilience of Northern Field Crop Systems Using Inter-Seeded Red Clover: A Review
Abstract
:1. Introduction

2. Current Red Clover Establishment Practices
2.1. Variety Selection, Seeding Date and Rate
2.2. Cereal Management
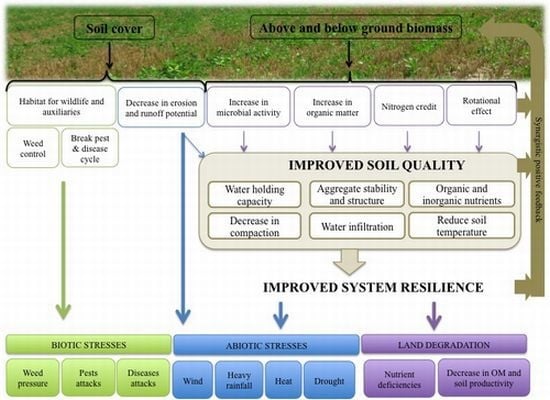
3. Potential of Inter-seeded Red Clover to Mitigate Land Degradation
3.1. Direct Biomass Contribution to Soil Organic Matter
3.1.1. Biomass Production
| Location | Above ground biomass (Mg ha−1) | Sources | |
|---|---|---|---|
| Fall | Spring | ||
| Ontario | 0.69–4.02 | 1.71–5.33 | [15,35,71,83,84,85] |
| Michigan | 1.51–3.0 | [58] | |
| Wisconsin | 0.86–2.08 | [86] | |
| Iowa | 0.0–6.52 (1) | [65,67,70,80,87] | |
| Saskatchewan | 1.1–1.3 | [88] | |
| Manitoba | 0.6–1.8 | [54] | |
3.1.2. Decomposition and Mineralization Rates
3.1.3. Contribution to Soil Organic Matter
3.2. Red Clover Effects on Subsequent Crop Biomass Production
| Tillage system | Maize price 1 | N cost | Cover crop | MERN 2 | MEY 3 | Gross return 4 | Profit |
|---|---|---|---|---|---|---|---|
| $ Mg−1 | $ Kg−1 | Kg N ha−1 | Mg ha−1 | $ ha−1 | |||
| No-till | 150 | 1 | No red clover | 209 | 9803 | 1278 | |
| Red clover | 146 | 9899 | 1316 | ||||
| Difference | ** | ns | ns | 38 | |||
| 100 | 1 | No red clover | 189 | 9631 | 791 | ||
| Red clover | 125 | 9719 | 825 | ||||
| Difference | ** | ns | ns | 33 | |||
| 150 | 1.5 | No red clover | 189 | 9631 | 1187 | ||
| Red clover | 125 | 9719 | 1257 | ||||
| Difference | ** | ns | ns | 70 | |||
| 100 | 1.5 | No red clover | 158 | 9250 | 713 | ||
| Red clover | 101 | 9421 | 778 | ||||
| Difference | ** | ns | ns | 64 | |||
| Conventional tillage | 150 | 1 | No red clover | 143 | 9454 | 1293 | |
| Red clover | 79 | 9886 | 1382 | ||||
| Difference, | ** | ** | ** | 89 | |||
| Rotational effect (%) | 4.57% | ||||||
| 100 | 1 | No red clover | 129 | 9338 | 822 | ||
| Red clover | 74 | 9841 | 888 | ||||
| Difference | ** | ** | ** | 66 | |||
| Rotational effect (%) | 5.38% | ||||||
| 150 | 1.5 | No red clover | 129 | 9338 | 1234 | ||
| Red clover | 74 | 9841 | 1352 | ||||
| Difference | ** | ** | ** | 118 | |||
| Rotational effect (%) | 5.38% | ||||||
| 100 | 1.5 | No red clover | 107 | 9068 | 772 | ||
| Red clover | 63 | 9713 | 863 | ||||
| Difference | ** | ** | ** | 90 | |||
| Rotational effect (%) | 7.11% | ||||||
3.3. Improvement of Soil Quality
4. Improving Input Use Efficiencies
4.1. Red Clover Helps Improve System Water Use Efficiency
4.2. Red Clover Improves Nitrogen Use Efficiency
4.2.1. Effects on Amount and Timing of N Release in the System
4.2.2. Effects on N Losses from the System
5. Effects on Resilience to Environmental Stresses

5.1. Biotic Stresses
5.2. Abiotic Stresses
6. Factors Affecting Red Clover Adoption
6.1. Misconception about Effects of Inter-Seeded Red Clover on Cereal Yield
6.2. Initial Investments and Net Returns
6.3. Difficulties in Establishing Homogeneous Red Clover Stands
7. Future Prospects for Research
| Benefits | Drawbacks | Research needed | |
|---|---|---|---|
| Management practices and long term soil fertility |
|
|
|
| Profitability and adoption |
|
|
|
| Environmental sustainability and resilience |
|
|
|
8. Conclusions
Conflict of Interest
References
- USDA, World Agricultural Production: Monthly Circular Series for World Agricultural Production; USDA: Washington, DC, USA, 2012.
- USDA-NASS Commodity Statistics Database. USDA Web site. Available online: http://www.nass.usda.gov/Statistics_by_Subject/index.php?sector=CROPS (accessed on 5 November 2012).
- FAO Food Price Index Database. FAO Web site. Available online: http://www.fao.org/worldfoodsituation/wfs-home/foodpricesindex/en/ (accessed on 5 November 2012).
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis; Solomon, S.D., Qin, M., Manning, Z., Chen, M., Marquis, K.B., Averyt, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Scholberg, J.M.S.; Dogliotti, S.; Leoni, C.; Cherr, C.M.; Zotarelli, L.; Rossing, W.A.H. Cover Crops for Sustainable Agrosystems in the Americas. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 23–58. [Google Scholar]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P. Agricultural management practices to sustain crop yields and improve soil and environmental qualities. Sci. World J. 2003, 3, 768–789. [Google Scholar] [CrossRef]
- Rosenberg, N.J. Adaptation of agriculture to climate change. Clim. Chang. 1992, 21, 385–405. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and n dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Lal, R.; Regnier, E.; Eckert, D.J.; Edwards, W.M.; Hammond, R. Expectations of Cover Crops for Sustainable Agriculture. In Cover Crops for Clean Water; Hargrove, W.L., Ed.; Soil and Water Conservation Society: Ankeny, IA, USA, 1991; pp. 1–11. [Google Scholar]
- Lu, Y.-C.; Watkins, K.B.; Teasdale, J.R.; Abdul-Baki, A. Cover crops in sustainable food production. Food Rev. Int. 2000, 16, 121–157. [Google Scholar] [CrossRef]
- Dinnes, D.L.; Karlen, D.L.; Jaynes, D.B.; Kaspar, T.C. Review and Interpretation: Nitrogen Management Strategies to Reduce Nitrate Leaching in Tile-drained Midwestern Soils; Technical Report for USDA-ARS: Lincoln, NE, USA, 2002. [Google Scholar]
- Francis, G.; Bartley, K.; Tabley, F. The effect of winter cover crop management on nitrate leaching losses and crop growth. J. Agric. Sci. 1998, 131, 299–308. [Google Scholar] [CrossRef]
- Doran, J.W.; Smith, M.S. Role of Cover Crops in Nitrogen Cycling. In Cover Crops for Clean Water; Hargrove, W.L., Ed.; Soil and Water Conservation Society: Ankeny, IA, USA, 1991; pp. 85–90. [Google Scholar]
- Langdale, G.W.; Blevins, R.L.; Karlen, D.L.; Mccool, D.K.; Nearing, M.A.; Skidmore, E.L.; Thomas, A.W.; Tyler, D.D.; Williams, J.R. Cover Crop Effects on Soil Erosion by Wind and Water. In Cover Crops for Clean Water; Hargrove, W.L., Ed.; Soil and Water Conservation Society: Ankeny, IA, USA, 1991; pp. 15–22. [Google Scholar]
- Dapaah, H.K.; Vyn, T.J. Nitrogen fertilization and cover crop effects on soil structural stability and corn performance. Commun. Soil Sci. Plant Anal. 1998, 29, 2557–2569. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Radke, J.K.; Laflen, J.M. Small grain cover crops and wheel traffic effects on infiltration, runoff, and erosion. J. Soil Water Conserv. 2001, 56, 160–164. [Google Scholar]
- Reicosky, D.C.; Forcella, F. Cover crop and soil quality interactions in agroecosystems. J. Soil Water Conserv. 1998, 53, 224–229. [Google Scholar]
- Willson, T.C.; Paul, E.A.; Harwood, R.R. Biologically active soil organic matter fractions in sustainable cropping systems. Appl. Soil Ecol. 2001, 16, 63–76. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S.; Stephen, R.C. Influence of mixed cropping rotations (pasture-arable) on organic matter content, water stable aggregation and clod porosity in a group of soils. Soil Tillage Res. 1991, 19, 77–87. [Google Scholar] [CrossRef]
- Hu, S.; Grunwald, N.J.; van Bruggen, A.H.C.; Gamble, G.R.; Drinkwater, L.E.; Shennan, C.; Demment, M.W. Short-term effects of cover crop incorporation on soil carbon pools and nitrogen availability. Soil Sci. Soc. Am. J. 1997, 61, 901–911. [Google Scholar] [CrossRef]
- Carter, M.R.; Kunelius, H.T. Effect of undersowing barley with annual ryegrasses or red clover on soil structure in a barley-soybean rotation. Agric. Ecosyst. Environ. 1993, 43, 245–254. [Google Scholar]
- Fisk, J.W.; Hesterman, O.B.; Shrestha, A.; Kells, J.J.; Harwood, R.R.; Squire, J.M.; Sheaffer, C.C. Weed suppression by annual legume cover crops in no-tillage corn. Agron. J. 2001, 93, 319–325. [Google Scholar] [CrossRef]
- Singer, J.; Cox, W.J.; Hahn, R.R.; Shields, E.J. Cropping system effects on weed emergence and densities in corn. Agron. J. 2000, 92, 754–760. [Google Scholar] [CrossRef]
- Hiltbrunner, J.; Liedgens, M.; Bloch, L.; Stamp, P.; Streit, B. Legume cover crops as living mulches for winter wheat: Components of biomass and the control of weeds. Eur. J. Agron. 2007, 26, 21–29. [Google Scholar] [CrossRef]
- Mutch, D.R.; Martin, T.E.; Kosola, K.R. Red clover (Trifolium pratense) suppression of common ragweed (Ambrosia) in winter wheat (Triticum aestivum). Weed Technol. 2003, 17, 181–185. [Google Scholar] [CrossRef]
- Raimbault, B.A.; Vyn, T.J. Crop rotation and tillage effects on corn growth and soil structural stability. Agron. J. 1991, 83, 979–985. [Google Scholar] [CrossRef]
- Unger, P.W.; Merle, F. Cover crop effects on soil water relationships. J. Soil Water Conserv. 1998, 53, 200–207. [Google Scholar]
- Sarrantonio, M.; Gallandt, E. The role of cover crops in north American cropping systems. J. Crop Prod. 2003, 8, 53–74. [Google Scholar] [CrossRef]
- Varco, J.; Frye, W.; Smith, M.; MacKown, C. Tillage effects on nitrogen recovery by corn from a nitrogen-15 labeled legume cover crop. Soil Sci. Soc. Am. J. 1989, 53, 822–827. [Google Scholar] [CrossRef]
- McVay, K.; Radcliffe, D.; Hargrove, W. Winter legume effects on soil properties and nitrogen fertilizer requirements. Soil Sci. Soc. Am. J. 1989, 53, 1856–1862. [Google Scholar]
- Kuo, S.; Sainju, U. Nitrogen mineralization and availability of mixed leguminous and non-leguminous cover crop residues in soil. Biol. Fertil. Soils 1998, 26, 346–353. [Google Scholar] [CrossRef]
- Vicia, L.; Roth, V. Winter legumes as a nitrogen source for no-till grain sorghum. Agron. J. 1986, 78, 70–74. [Google Scholar]
- Oyer, L.J.; Touchton, J.T. Utilizing legume cropping systems to reduce nitrogen fertilizer requirements for conservation-tilled corn. Agron. J. 1990, 82, 1123–1127. [Google Scholar] [CrossRef]
- Odhiambo, J.; Bomke, A. Grass and legume cover crop effects on dry matter and nitrogen accumulation. Agron. J. 2001, 93, 299–307. [Google Scholar] [CrossRef]
- Vyn, T.J.; Faber, J.G.; Janovicek, K.J.; Beauchamp, E.G. Cover crop effects on nitrogen availability to corn following wheat. Agron. J. 2000, 92, 915–924. [Google Scholar]
- Bruulsema, T.W.; Christie, B.R. Nitrogen contribution to succeeding corn from alfalfa and red clover. Agron. J. 1987, 79, 96–100. [Google Scholar] [CrossRef]
- Pieters, A. Green Manuring, Principles and Practice; John Wiley & Sons Inc: New York, NY, USA, 1927; p. 356. [Google Scholar]
- Singer, J. Corn Belt assessment of cover crop management and preferences. Agron. J. 2008, 100, 1670–1672. [Google Scholar] [CrossRef]
- Singer, J.W.; Nusser, S.M.; Alf, C.J. Are cover crops being used in the US Corn Belt? J. Soil Water Conserv. 2007, 62, 353–358. [Google Scholar]
- Crop Harvested Area 2010. FAO FAOSTAT Web site. Available online: http://faostat3.fao.org/home/index.html (accessed on 5 November 2012).
- Liebig, M.A.; Varvel, G.E.; Doran, J.W.; Wienhold, B.J. Crop sequence and nitrogen fertilization effects on soil properties in the western Corn Belt. Soil Sci. Soc. Am. J. 2002, 66, 596–601. [Google Scholar] [CrossRef]
- Hussain, S.K.; Mlelke, L.N.; Skopp, J. Detachment of soil as affected by fertility management and crop rotations. Soil Sci. Soc. Am. J. 1988, 52, 1463–1468. [Google Scholar] [CrossRef]
- Fahad, A.A.; Mielke, L.N.; Flowerday, A.D.; Swartzendruber, D. Soil physical properties as affected by soybean and other cropping sequences. Soil Sci. Soc. Am. J. 1982, 46, 377–381. [Google Scholar] [CrossRef]
- Miles, R.J.; Brown, J.R. The sanborn field experiment: Implications for long-term soil organic carbon levels. Agron. J. 2011, 103, 268–278. [Google Scholar]
- Studdert, G.; Echeverria, H. Crop rotations and nitrogen fertilization to manage soil organic carbon dynamics. Soil Sci. Soc. Am. J. 2000, 64, 1496–1503. [Google Scholar] [CrossRef]
- Varvel, G.E. Rotation and nitrogen fertilization effects on changes in soil carbon and nitrogen. Agron. J. 1994, 86, 319–325. [Google Scholar] [CrossRef]
- Havlin, J.; Kissel, D.; Maddux, L.; Claassen, M.; Long, J. Crop rotation and tillage effects on soil organic carbon and nitrogen. Soil Sci. Soc. Am. J. 1990, 54, 448–452. [Google Scholar] [CrossRef]
- Yamoah, C.F.; Varvel, G.E.; Waltman, W.J.; Francis, C.A. Long-term nitrogen use and nitrogen-removal index in continuous crops and rotations. Field Crop Res. 1998, 57, 15–27. [Google Scholar]
- Varvel, G.E.; Peterson, T.A. Residual soil nitrogen as affected by continuous, two-year, and four-year crop rotation systems. Agron. J. 1990, 82, 958–962. [Google Scholar] [CrossRef]
- Miller, D.R.; Chen, S.Y.; Porter, P.M.; Johnson, G.A.; Wyse, D.L.; Stetina, S.R.; Klossner, L.D.; Nelson, G.A. Rotation crop evaluation for management of the soybean cyst nematode in Minnesota. Agron. J. 2006, 98, 569–578. [Google Scholar] [CrossRef]
- Snapp, S.S.; Swinton, S.M.; Labarta, R.; Mutch, D.; Black, J.R.; Leep, R.; Nyiraneza, J. Evaluating cover crops for benefits, costs and performance within cropping system niches. Agron. J. 2005, 97, 322–332. [Google Scholar]
- Fowler, D.B. Wheat production in the high winter stress climate of the great plains of north America—An experiment in crop adaptation. Crop Sci. 2012, 52, 11–20. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Molnar, L.J.; Moyer, J.R. Suitability of legume cover crop-winter wheat intercrops on the semi-arid Canadian prairies. Can. J. Plant Sci. 2010, 90, 479–488. [Google Scholar] [CrossRef]
- Martens, J.R.T.; Hoeppner, J.W.; Entz, M.H. Legume cover crops with winter cereals in southern Manitoba: Establishment, productivity, and microclimate effects. Agron. J. 2001, 93, 876–883. [Google Scholar]
- Hesterman, O.B.; Griffin, T.S.; Willimas, P.T.; Harris, G.H.; Christenson, D.R. Forage Legume-small grain intercrops: Nitrogen production and response of subsequent corn. J. Prod. Agric. 1992, 5, 340–348. [Google Scholar]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen fixation of red clover interseeded with winter cereals across a management-induced fertility gradient. Nutr. Cycl. Agroecos. 2010, 90, 105–119. [Google Scholar]
- Hartl, W. Influence of undersown clovers on weeds and on the yield of winter wheat in organic farming. Agric. Ecosyst. Environ. 1989, 27, 389–396. [Google Scholar] [CrossRef]
- Tiffin, P.; Hesterman, O.B. Response of corn grain yield to early and late killed red clover green manure and subirrigation. J. Prod. Agric. 1998, 11, 112–121. [Google Scholar]
- Stute, J.K.; Posner, J.L. Legume cover crop options for grain rotations in Wisconsin. Agron. J. 1993, 85, 1128–1132. [Google Scholar] [CrossRef]
- Fertilizer Use and Price Index. USDA-ERS Web site. Available online: http://www.ers.usda.gov/data-products/fertilizer-use-and-price.aspx (accessed on 5 November 2012).
- Deen, B. University of Guelph: Guelph, Canada, 2012; Personal Communication.
- Gibson, L.; Singer, J.; Barnhart, S.; Blaser, B. Intercropping Winter Cereal Grains and Red Clover; Technical Report PM 2025 for Iowa State University Extension: Ames, IA, USA, 2006. [Google Scholar]
- Clark, A. Red Clover. In Managing Cover Crops Profitably; Sustainable Agriculture Network: Beltsville, MD, USA, 2007; pp. 159–164. [Google Scholar]
- Madill, J.; Skepasts, A. Red clover in Ontario; Technical Report No. 81-080; Ontario Ministry of Agriculture and Food: Toronto, Canada, 1981. [Google Scholar]
- Singer, J.; Casler, M.D.; Kohler, K.A. Wheat effect on frost-seeded red clover cultivar establishment and yield. Agron. J. 2006, 98, 265–269. [Google Scholar] [CrossRef]
- Meyer, D.W.; Badaruddin, M. Frost tolerance of ten seedling legume species at four growth stages. Crop Sci. 2001, 41, 1838–1842. [Google Scholar] [CrossRef]
- Blaser, B.C.; Gibson, L.R.; Singer, J.W.; Jannink, J.-L. Optimizing seeding rates for winter cereal grains and frost-seeded red clover intercrops. Agron. J. 2006, 98, 1041–1049. [Google Scholar] [CrossRef]
- Blaser, B.C.; Singer, J.W.; Gibson, L.R. Winter cereal, seeding rate, and intercrop seeding rate effect on red clover yield and quality. Agron. J. 2007, 99, 723–729. [Google Scholar] [CrossRef]
- Singer, J.; Meek, D.W. Relationship between interseeded red clover biomass and plant number. Crop Sci. 2012, 52, 981–985. [Google Scholar]
- Blaser, B.C.; Singer, J.W.; Gibson, L.R. Winter cereal canopy effect on cereal and interseeded legume productivity. Agron. J. 2011, 103, 1180–1185. [Google Scholar] [CrossRef]
- Queen, A.; Earl, H.; Deen, W. Light and moisture competition effects on biomass of red clover underseeded to winter wheat. Agron. J. 2009, 101, 1511–1521. [Google Scholar] [CrossRef]
- Garand, M.J.; Simard, R.R.; MacKenzie, A.F.; Hamel, C. Underseeded clover as a nitrogen source for spring wheat on a gleysol. Can. J. Soil Sci. 2001, 81, 93–102. [Google Scholar]
- Kunelius, H.T.; Johnston, H.W.; Macleod, J.A. Effect of undersowing barley with Italian ryegrass or red clover on yield, crop composition and root biomass. Agric. Ecosyst. Environ. 1992, 38, 127–137. [Google Scholar] [CrossRef]
- Katsvairo, T.; Cox, W.J.; van Es, H. Tillage and rotation effects on soil physical characteristics. Agron. J. 2002, 94, 121–125. [Google Scholar]
- Drury, C.F.; Tan, C.-S.; Welacky, T.W.; Oloya, T.O.; Hamill, A.S.; Weaver, S.E. Red clover and tillage influence on soil temperature, water content, and corn emergence. Agron. J. 1999, 91, 101–108. [Google Scholar] [CrossRef]
- Brandt, J.E.; Hons, F.M.; Haby, A. Effects of subterranean clover interseeding on grain yield, yield components, and nitrogen content of soft red winter wheat. J. Prod. Agric. 1989, 2, 347–351. [Google Scholar]
- Singer, J.; Cox, W.J. Corn growth and yield under different crop rotation, tillage, and management systems. Crop Sci. 1998, 38, 996–1003. [Google Scholar] [CrossRef]
- Legere, A.; Stevenson, F.C.; Samson, N. Tillage and weed management effects on forage production in a barley-red clover rotation. Can. J. Plant Sci. 2001, 81, 405–412. [Google Scholar]
- Fribourg, H.A.; Johnson, I.J. Dry matter and nitrogen yields of legume tops and roots in the fall of the seeding year. Agron. J. 1955, 47, 73–77. [Google Scholar]
- Blaser, B.C.; Singer, J.W.; Gibson, L.R. Winter wheat and red clover intercrop response to tillage and compost amendment. Crop Sci. 2012, 52, 320–326. [Google Scholar] [CrossRef]
- Deen, B.; Earl, H. Impact of Winter Wheat Management on Underseeded Red Clover. In Proceedings of the American Society of Agronomy International Meeting, Indianapolis, IN, USA, 12–16 November 2006.
- Nass, H.G.; Papadopolous, Y.; MacLeod, J.A.; Caldwell, C.D.; Walker, D.F. Nitrogen management of spring milling wheat underseeded with red clover. Can. J. Plant Sci. 2002, 82, 653–659. [Google Scholar] [CrossRef]
- Vyn, T.J.; Janovicek, K.J.; Miller, M.; Beauchamp, E. Soil nitrate accumulation and corn response to preceding small-grain fertilization and cover crops. Agron. J. 1999, 91, 17–24. [Google Scholar] [CrossRef]
- Janovicek, K.J.; Vyn, T.J.; Voroney, R. No-till corn response to crop rotation and in-row residue placement. Agron. J. 1997, 89, 588–596. [Google Scholar] [CrossRef]
- Serran, S. Nitrogen Dynamics and Corn Growth in Manure/Cover Crop Systems. Ph.D. Dissertation, University of Guelph, Guelph, Canada, 2005. [Google Scholar]
- Stute, J.K.; Posner, J.L. Synchrony between legume nitrogen release and corn demand in the upper Midwest. Agron. J. 1995, 87, 1063–1069. [Google Scholar] [CrossRef]
- Singer, J.; Sauer, T.J.; Blaser, B.C.; Meek, D.W. Radiation use efficiency in dual winter cereal–forage production systems. Agron. J. 2007, 99, 1175–1179. [Google Scholar] [CrossRef]
- Bowren, K.; Cooke, D.; Downey, R. Yield of dry matter and nitrogen from tops and roots of sweetclover, alfalfa and red clover at five stages of growth. Can. J. Plant Sci. 1969, 49, 61–68. [Google Scholar] [CrossRef]
- Bray, J.R. Root production and the estimation of net productivity. Can. J. Bot. 1963, 41, 65–72. [Google Scholar] [CrossRef]
- Bolinder, M.A.; Angers, D.A.; Bélanger, G.; Michaud, R.; Laverdière, M.R. Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can. J. Plant Sci. 2002, 82, 731–737. [Google Scholar] [CrossRef]
- Mallory, E.B.; Posner, J.L.; Baldock, J.O. Performance, economics, and adoption of cover crops in Wisconsin cash grain rotations: On-farm trials. Am. J. Altern. Agric. 1998, 13, 2–11. [Google Scholar] [CrossRef]
- Sarrantonio, M.; Scott, T. Tillage effects on availability of nitrogen to corn following a winter green manure crop. Soil Sci. Soc. Am. J. 1988, 52, 1661–1668. [Google Scholar] [CrossRef]
- Groffman, P.M.; Hendrix, P.F.; Crossley, D.A., Jr. Nitrogen dynamics in conventional and no-tillage agroecosystems with inorganic fertilizer or legume nitrogen inputs. Plant Soil 1987, 97, 315–332. [Google Scholar] [CrossRef]
- Dou, Z.; Fox, R.; Toth, J. Seasonal soil nitrate dynamics in corn as affected by tillage and nitrogen source. Soil Sci. Soc. Am. J. 1995, 59, 858–864. [Google Scholar]
- Haney, R.L.; Senseman, S.A.; Hons, F.M.; Zuberer, D.A. Effect of glyphosate on soil microbial activity and biomass. Weed Sci. 2000, 48, 89–93. [Google Scholar] [CrossRef]
- Haney, R.; Senseman, S.; Krutz, L.; Hons, F. Soil carbon and nitrogen mineralization as affected by atrazine and glyphosate. Biol. Fertil. Soils 2002, 35, 35–40. [Google Scholar]
- Damin, V.; Trivelin, P. Herbicides Effect on Nitrogen Cycling in Agroecosystems. In Herbicide and Environment; Kortekamp, A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Tradiff, F.; Smith, P. Red Clover Tolerance to Different Herbicide Application Timings; Ontario Ministry of Agriculture and Food: Toronto, Canada, 2005. [Google Scholar]
- Ebelhar, S.A.; Frye, W.W.; Blevins, R.L. Nitrogen from legume cover crops for no-tillage corn. Agron. J. 1984, 76, 51–55. [Google Scholar] [CrossRef]
- Wilson, D.; Hargrove, W. Release of nitrogen from crimson clover residue under two tillage systems. Soil Sci. Soc. Am. J. 1986, 50, 1251–1254. [Google Scholar]
- Ranells, N.N.; Wagger, M.G. Nitrogen-15 recovery and release by rye and crimson clover cover crops. Soil Sci. Soc. Am. J. 1997, 61, 943–948. [Google Scholar]
- Wagger, M.G. Cover crop management and nitrogen rate in relation to growth and yield of no-till corn. Agron. J. 1989, 81, 533–538. [Google Scholar]
- Drury, C.F.; Tan, C.S.; Reynolds, W.D.; Welacky, T.W.; Weaver, S.E.; Hamill, A.S.; Vyn, T.J. Impacts of zone tillage and red clover on corn performance and soil physical quality. Soil Sci. Soc. Am. J. 2003, 67, 867–877. [Google Scholar]
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- Rice, C.W.; Grove, J.H.; Smith, M.S. Estimating soil net nitrogen mineralization as affected by tillage and soil drainage due to topographic position. Can. J. Soil Sci. 1987, 67, 513–520. [Google Scholar]
- Davis, A.S.; Hill, J.D.; Chase, C.A.; Johanns, A.M.; Liebman, M. Increasing cropping system diversity balances productivity, profitability and environmental health. PloS One 2012, 7, 1–8. [Google Scholar]
- Meyer-Aurich, A.; Janovicek, K.; Deen, W.; Weersink, A. Impact of tillage and rotation on yield and economic performance in corn-based cropping systems. Agron. J. 2006, 98, 1204–1212. [Google Scholar]
- Janzen, H.H.; Bole, J.B.; Biederbeck, V.O.; Slinkard, A.E. Fate of N applied as green manure of ammonium sulphate fertilizer to soil subsequently cropped with spring wheat in three sites in western Canada. Can. J. Soil Sci. 1990, 70, 313–323. [Google Scholar]
- Ladd, J.N.; Amato, M.; Jackson, R.B.; Butler, J.H.A. Utilization by wheat crops of nitrogen from legume residues decomposing in the field. Soil Biol. Biochem. 1983, 15, 231–238. [Google Scholar]
- Drury, C.; Stone, J.; Findlay, W. Microbial biomass and soil structure associated with corn, grasses, and legumes. Soil Sci. Soc. Am. J. 1991, 55, 805–811. [Google Scholar]
- Adams, W.; Morris, H.D.; Dawson., R.N. Effect of cropping systems and nitrogen levels on corn (Zea mays) yields in the southern piedmont region. Agron. J. 1970, 62, 655–659. [Google Scholar] [CrossRef]
- Henry, D.C.; Mullen, R.W.; Dygert, C.E.; Diedrick, K.A.; Sundermeier, A. Nitrogen contribution from red clover for corn following wheat in western Ohio. Agron. J. 2010, 102, 210–215. [Google Scholar] [CrossRef]
- Stanger, T.F.; Lauer, J.G. Corn grain yield response to crop rotation and nitrogen over 35 years. Agron. J. 2008, 100, 643–650. [Google Scholar] [CrossRef]
- Bolton, E.F.; Dirks, V.A.; Findlay, W.I. Some relationships between soil porosity, leaf nutrient composition and yield for certain corn rotations at two fertility levels on Brookston clay. Can. J. Plant Sci. 1979, 59, 1–9. [Google Scholar] [CrossRef]
- Miguez, F.E.; Bollero, G.A. Review of corn yield response under winter cover cropping systems using meta-analytic methods. Crop Sci. 2005, 45, 2318–2329. [Google Scholar] [CrossRef]
- Hively, W.D.; Cox, W.J. Interseeding cover crops into soybean and subsequent corn yields. Agron. J. 2001, 93, 308–313. [Google Scholar] [CrossRef]
- Benson, G.O. Why the Reduced Yields When Corn Follows Corn and Possible Management Responses. In Proceedings of the Corn and Sorghum Research Conference, Chicago, IL, USA, 11–13 December 1985; pp. 161–174.
- Copeland, P.; Crookston, P. Crop sequence affects nutrient composition of corn and soybean grown under high fertility. Agron. J. 1992, 84, 503–509. [Google Scholar] [CrossRef]
- Copeland, J.; Allmaras, R.R.; Crookston, R.K.; Nelson, W.W. Corn-soybean rotation effects on soil water depletion. Agron. J. 1993, 85, 203–210. [Google Scholar] [CrossRef]
- Johnson, N.C.; Copeland, P.J.; Crookston, R.K.; Pfleger, F.L. Mycorrhizae: Possible explanation for yield decline with continuous corn and soybean. Agron. J. 1992, 84, 387–390. [Google Scholar] [CrossRef]
- Corak, S.J.; Frye, W.W.; Smith, M.S. Legume mulch and nitrogen fertilizer effects on soil water and corn production. Soil Sci. Soc. Am. J. 1991, 55, 1395–1400. [Google Scholar] [CrossRef]
- Utomo, M.; Frye, W.W.; Blevins, R.L. Sustaining soil nitrogen for corn using hairy vetch cover crop. Agron. J. 1990, 82, 979–983. [Google Scholar] [CrossRef]
- Deen, B.; Janovicek, K.; Stewart, G. Influence of Red Clover on Maize Nitrogen Recommendations in Eastern Canada. In Proceedings of IX European Society for Agronomy Congress, Warsaw, Poland, 4–7 September 2006.
- Janovicek, K.; Stewart, G.A. Updating General Fertilizer Nitrogen Recommendations for Corn in Ontario. In Proceedings of the 34th North Central Extension-Industry Soil Fertility Conference, Des Moines, IA, USA, 17–18 November 2004; pp. 12–19.
- Angers, D.A.; Mehuys, G.R. Aggregate Stability to Water. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 651–658. [Google Scholar]
- Stone, J.; Butterly, B. Nine forages and the aggreagtion of a clay loam soil. Can. J. Soil Sci. 1989, 69, 165–169. [Google Scholar]
- Six, J.; Elliott, E.T.; Paustian, K. Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci. Soc. Am. J. 1999, 63, 1350–1358. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Broersma, K.; Robertson, J.A.; Chanasyk, D.S. The effects of diverse cropping systems on aggregation of a luvisolic soil in the peace river region. Can. J. Soil Sci. 1997, 77, 323–329. [Google Scholar]
- Lynch, J.M.; Bragg, E. Microorganisms and Soil Aggregate Stability. In Advances in Soil Science; Stewart, B.A., Ed.; Spriger: New York, NY, USA, 1985; Volume 2, pp. 133–171. [Google Scholar]
- Baldock, J.A.; Kay, B.D. Influence of cropping history and chemical treatments on the water-stable aggregation of a silt loam. Can. J. Soil Sci. 1987, 67, 501–511. [Google Scholar] [CrossRef]
- Morin, J.; Benyamini, Y.; Michaeli, A. The effect of raindrop impact on the dynamics of soil surface crusting and water movement in the profile. J. Hydrol. 1981, 52, 321–335. [Google Scholar] [CrossRef]
- Carter, M.R. Soil quality for sustainable land management: Organic matter and aggregation interactions that maintain soil function. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Hudson, B. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Allison, F.E. Soil Organic Matter and Its Role in Crop Production; Elsevier: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter; FAO: Rome, Italy, 2005. [Google Scholar]
- Patriquin, D. Water, Soil and Organic Matter: A Complex Relationship. The Canadian Organic Grower, Fall 2004. Available online: http://www.cog.ca/documents/Water.pdf (accessed on 9 November 2012).
- Osborne, S.; Schumacher, T.; Humburg, D. Evaluation of cover crops to increase corn emergence, yield and field trafficability. Agric. J. 2008, 3, 397–400. [Google Scholar]
- Reynolds, W.D.; Bowman, B.T.; Brunke, R.R.; Drury, C.F.; Tan, C.S. Comparison of tension infiltrometer, pressure infiltrometer, and soil core estimates of saturated hydraulic conductivity. Soil Sci. Soc. Am. J. 2000, 64, 478–484. [Google Scholar] [CrossRef]
- Kanneganti, V.R.; Kaffka, S.R. Forage availability from a temperate pasture managed with intensive rotational grazing. Grass Forage Sci. 1995, 50, 55–62. [Google Scholar] [CrossRef]
- Ewing, R.P.; Wagger, M.G.; Denton, H.P. Tillage and cover crop management effects on soil water and corn yield. Soil Sci. Soc. Am. J. 1991, 55, 1081–1085. [Google Scholar]
- Johnson, M.D.; Lowery, B. Effect of three conservation tillage practices on soil temperature and thermal properties. Soil Soc. Sci. Am. J. 1985, 49, 1547–1552. [Google Scholar]
- Alessi, J.; Power, J.F. Corn emergence in relation to soil temperature and seeding depth. Agron. J. 1971, 65, 717–719. [Google Scholar] [CrossRef]
- Lei, T.; Zhan, W.; Xiao, J.; Huang, X.; Mao, J. Water use efficiency of a mixed cropping system of corn with grasses. Int. J. Sustain. Dev. World Ecol. 2009, 12, 37–41. [Google Scholar]
- Russell, C.; Fillery, I. Estimates of lupin below-ground biomass nitrogen, dry matter, and nitrogen turnover to wheat. Aust. J. Agric. Res. 1996, 47, 1047–1059. [Google Scholar] [CrossRef]
- McNeill, A.; Zhu, C.; Fillery, I. Use of in situ 15N-labelling to estimate the total below-ground nitrogen of pasture legumes in intact soil–plant systems. Aust. J. Agric. Res. 1997, 8, 295–304. [Google Scholar]
- Sheard, R.W.; Bruulsema, T.W.; Christie, B.R. The Utilization of Nitrogen from 15N-Labelled Legume Residues by Barley. In Proceedings of the 29th National Alfalfa Improvement Conference, Lethbridge, Canada, 15–20 July 1984; p. 34.
- Harris, G.; Hcsterman, O.B.; Paul, E.A.; Peters, S.E. Fate of legume and fertilizer. Agron. J. 1990, 82, 910–915. [Google Scholar]
- Bergström, L.; Kirchmann, H. Leaching and crop uptake of nitrogen from nitrogen-15-labeled green manures and ammonium nitrate. J. Environ. Qual. 2004, 33, 1786–1792. [Google Scholar] [CrossRef]
- Gardner, J.B.; Drinkwater, L.E. The fate of nitrogen in grain cropping systems: A meta-analysis of 15N field experiments. Ecol. Appl. 2009, 19, 2167–2184. [Google Scholar]
- Yaacob, O.; Blair, G.J. Effect of legume cropping and organic matter accumulation on the infiltration rate and structure stability of a granite soil under a simulated topical environment. Plant Soil 1981, 60, 11–20. [Google Scholar] [CrossRef]
- Harris, G.H.; Hesterman, O.B. Quantifying the nitrogen contribution from alfalfa to soil and two succeeding crops using nitrogen-15. Agron. J. 1990, 82, 129–134. [Google Scholar] [CrossRef]
- Hanway, J.J. Growth stages of corn (Zea mays L.). Agron. J. 1963, 55, 487–492. [Google Scholar] [CrossRef]
- Burity, H.A.; Faris, M.A.; Ta, T.C.; Coulman, B. Fixation and transfer of nitrogen from legumes to grasses under mixed culture conditions. Plant Soil 1989, 114, 249–255. [Google Scholar] [CrossRef]
- Davies, D.B.; Sylvester-Bradley, R. The contribution of fertiliser nitrogen to leachable nitrogen in the UK: A review. J. Sci. Food Agric. 1995, 68, 399–406. [Google Scholar] [CrossRef]
- Boller, B.C.; Nosberger, J. Differences in nitrogen fixation among field-grown red clover strains at different levels of 15N fertilization. Euphytica 1994, 78, 167–174. [Google Scholar]
- Nesheim, L.; Øyen, J. Nitrogen fixation by red clover (Trifolium pratense L.) grown in mixtures with timothy (Phleum pratense L.) at different levels of nitrogen fertilization. Acta Agric. Scand. 1994, 44, 28–34. [Google Scholar]
- Liebman, M.; Graef, R.; Nettleton, D.; Cambardella, C.A. Use of legume green manures as nitrogen sources for corn production. Renew. Agric. Food Syst. 2012, 27, 180–191. [Google Scholar] [CrossRef]
- Dorland, S.; Beauchamp, E.G. Denitrification and ammonification at low soil temperature. Can. J. Soil Sci. 1991, 71, 293–303. [Google Scholar]
- Pelletier, F.; Prevost, D.; Laliberte, G.; van Bochove, E. Seasonal response of denitrifiers to temperature in a Quebec cropped soil. Can. J. Soil Sci. 1999, 79, 551–556. [Google Scholar] [CrossRef]
- Wagnar-Riddle, C.; Furon, A.; Mclaughlin, N.; Lee, I.; Barbeau, J.; Jayasundara, S.; Parkin, G.; Von Bertoldi, P.; Warland, J. Intensive measurement of nitrous oxide emissions from a corn-soybean-wheat rotation under two contrasting management systems over 5 years. Glob. Chang. Biol. 2007, 3, 1722–1736. [Google Scholar]
- Miller, M.N.; Zebarth, B.J.; Dandie, C.E.; Burton, D.L.; Goyer, C.; Trevors, J.T. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 2008, 40, 2553–2562. [Google Scholar] [CrossRef]
- Meyer Aurich, A.; Weersink, A.; Janovicek, K.; Deen, B. Cost efficient rotation and tillage options to sequester carbon and mitigate GHG emissions from agriculture in eastern Canada. Agric. Ecosyst. Environ. 2006, 117, 119–127. [Google Scholar] [CrossRef]
- Lynch, D. Environmental impacts of organic agriculture: A Canadian perspective. Can. J. Plant Sci. 2009, 3, 621–628. [Google Scholar] [CrossRef]
- Holling, C. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar]
- Teasdale, J.; Brandsæter, L.; Calegari, A.; Neto, F.S. Cover Crops and Weed Management. In Non-Chemical Weed Management: Principles, Concepts and Techology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CABI International: Oxfordshire, UK, 2007; pp. 49–64. [Google Scholar]
- Nelson, W.A.; Kahn, B.A.; Roberts, B.W. Screening cover crops for use in conservation tillage systems for vegetables following spring plowing. Hortic. Sci. 1991, 26, 860–862. [Google Scholar]
- Blackshaw, R.E.; Moyer, J.R.; Doram, R.C.; Boswall, A.L.; Smith, E.G.; Linum, L. Suitability of undersown sweetclover as a fallow replacement in semiarid cropping systems. Agron. J. 2001, 93, 863–868. [Google Scholar] [CrossRef]
- Brandsæter, L.O.; Goul Thomsen, M.; Wærnhus, K.; Fykse, H. Effects of repeated clover undersowing in spring cereals and stubble treatments in autumn on Elymus repens, Sonchus arvensis and Cirsium arvense. Crop Prot. 2012, 32, 104–110. [Google Scholar] [CrossRef]
- Ross, S.M.; King, J.R.; Izaurralde, R.C.; Donovan, J.T.O. Weed suppression by seven clover species. Agron. J. 2001, 93, 820–827. [Google Scholar] [CrossRef]
- Power, J. Legume and Crop Rotations. In Sustainable Agriculture in Temperate Zones; Francis, C.A., Butler Flora, C., King, L.D., Eds.; John Wisley & Sons, Inc.: New York, NY, USA, 1990; pp. 178–204. [Google Scholar]
- Liebman, M.; Davis, A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Chen, S.; Wyse, D.L.; Johnson, G.A.; Porter, P.M.; Stetina, S.R.; Miller, D.R.; Betts, K.J.; Klossner, L.D.; Haar, M.J. Effect of cover crops alfalfa, red clover, and perennial ryegrass on soybean cyst nematode population and soybean and corn yields in minnesota. Crop Sci. 2006, 46, 1890–1897. [Google Scholar] [CrossRef]
- Branson, T.F.; Ortman, E.E. The host range of larvae of the western corn rootworm: Further studies. J. Econ. Entomol. 1970, 63, 800–803. [Google Scholar]
- Nicholson, A.G.; Wien, H.C. Screening of turfgrass and clovers for use as living mulches in sweet corn and cabbage. J. Am. Soc. Hortic. Sci. 1983, 108, 1071–1076. [Google Scholar]
- Skarphol, B.J.; Corey, K.A.; Meisinger, J.J. Response of snap beans to tillage and cover crop combinations. J. Am. Soc. Hortic. Sci. 1987, 112, 936–941. [Google Scholar]
- Boyd, N.S.; Gordon, R.; Asiedu, S.K.; Martin, R.C. The effects of living mulches on tuber yields of potato (Solanum tuberosum L.). Biol. Agric. Hortic. 2001, 18, 203–220. [Google Scholar] [CrossRef]
- Martin, R.C.; Greyson, P.R.; Gordon, R. Competition between corn and a living mulch. Can. J. Plant Sci. 1999, 79, 579–586. [Google Scholar]
- Tisdall, J.; Oades, J. Stabilization of soil aggregates by the root systems of ryegrass. Aust. J. Soil Res. 1979, 17, 429–441. [Google Scholar] [CrossRef]
- Curaqueo, G.; Acevedo, E.; Cornejo, P.; Seguel, A.; Rubio, R.; Borie, F. Tillage effect on soil organic matter, mycorrhizal hyphae and aggregates in a mediterranean agroecosystem. Revista de la ciencia del suelo y nutrición vegetal 2010, 10, 12–21. [Google Scholar]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; de Carvalho, M. Effect of tillage and crop on arbuscular mycorrhiza colonization of winter wheat and triticale under Mediterranean conditions. Soil Use Manag. 2012, 28, 202–208. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Anken, T.; Ruh, R.; Sanders, I.R.; Frossard, E. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 2002, 12, 225–234. [Google Scholar] [CrossRef]
- Deguchi, S.; Shimazaki, Y.; Uozumi, S.; Tawaraya, K.; Kawamoto, H.; Tanaka, O. White clover living mulch increases the yield of silage corn via arbuscular mycorrhizal fungus colonization. Plant Soil 2007, 291, 291–299. [Google Scholar] [CrossRef]
- Lehman, R.M.; Taheri, W.I.; Osborne, S.L.; Buyer, J.S.; Douds, D.D. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 2012, 61, 300–304. [Google Scholar] [CrossRef]
- Tobar, R.M.; Azcon, R.; Barea, J.M. The improvement of plant n acquisition from an ammonium-treated, drought-stressed soil by the fungal symbiont in arbuscular mycorrhizae. Mycorrhiza 1994, 4, 105–108. [Google Scholar] [CrossRef]
- Karaki, G.N.; Clark, R.B. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J. Plant Nutr. 1998, 21, 263–276. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreall, C.M.; Ellerta, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar]
- Griffith, D.R.; Kladivko, E.J.; Mannering, J.V.; West, T.D.; Parsons, S.D. Long-term tillage and rotation effects on corn growth and yield on high and low organic matter, poorly drained soils. Agron. J. 1988, 80, 599–605. [Google Scholar] [CrossRef]
- Arbuckle, G.; Ferrell, J. Attitudes toward Cover Crops in Iowa: Benefits and Barriers; Technical Report for Iowa State University Extension PMR 1010; Iowa department of Agriculture and Land Stewarship: Ames, IA, USA, 2012. [Google Scholar]
- Ngalla, C.F.; Eckert, D.J. Wheat-Red Clover Interseeding As a Nitrogen Source For Corn. In Proceedings of the Annual Conference of the Soil and Water Conservation Society of America, Ankeny, IA, USA, 5 August 1987; pp. 47–48.
- Jones, L.; Clements, R. Development of a low input system for growing wheat (Triticum vulgare) in a permanent understorey of white clover (Trifolium repens). Ann. Appl. Biol. 1993, 123, 109–119. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Canadian farm fuel and fertilizer: Prices and expenses. Market Outlook Rep. 2010, 2, 1–8.
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Fuhrer, J. Agroecosystem responses to combinations of elevated CO2, ozone, and global climate change. Agric. Ecosyst. Environ. 2003, 97, 1–20. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gaudin, A.C.M.; Westra, S.; Loucks, C.E.S.; Janovicek, K.; Martin, R.C.; Deen, W. Improving Resilience of Northern Field Crop Systems Using Inter-Seeded Red Clover: A Review. Agronomy 2013, 3, 148-180. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy3010148
Gaudin ACM, Westra S, Loucks CES, Janovicek K, Martin RC, Deen W. Improving Resilience of Northern Field Crop Systems Using Inter-Seeded Red Clover: A Review. Agronomy. 2013; 3(1):148-180. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy3010148
Chicago/Turabian StyleGaudin, Amélie C. M., Sabrina Westra, Cora E. S. Loucks, Ken Janovicek, Ralph C. Martin, and William Deen. 2013. "Improving Resilience of Northern Field Crop Systems Using Inter-Seeded Red Clover: A Review" Agronomy 3, no. 1: 148-180. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy3010148