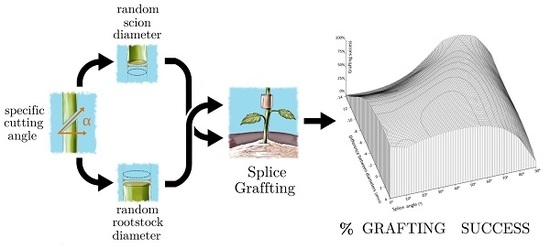
Combined Influence of Cutting Angle and Diameter Differences between Seedlings on the Grafting Success of Tomato Using the Splicing Technique
Abstract
:1. Introduction
- The meristematic tissues of the stem are placed in direct contact with the tissues of the rootstock. Once both components of the graft are in intimate contact, cambium cells from the rootstock and the scion produce parenchyma cells that fuse forming a callus tissue [9]. This first phase of cohesion that forms the callus is a reaction similar to wound healing, and it does not require recognition between rootstock and scion, occurring in both compatible and incompatible grafts.
- If the graft is compatible, a differentiation of certain vessels and sieve tube elements of the phloem is observed in the callus, and they are not derived from the cambium and constitute the first transitional and continuous union between the rootstock and the scion.
- In the last part of the grafting process, the cambium layer newly formed in the bridge of the callus begins its own meristematic tissue activity, thus forming new vascular tissue. Production of these new vascular elements that join xylem and phloem allows establishing a symplastic communication between rootstock and scion [1].
2. Materials and Methods
2.1. Definition of Operating Conditions
2.2. Device Description
3. Results and Discussion
3.1. Data Analysis: Descriptive Statistics
3.2. Data Analysis: ANOVA
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pina, A.Y.; Errea, P. A review of new advances in mechanism of graft compatibility–incompatibility. Sci. Hortic. 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Mudge, K.; Janick, J.; Scoffield, S.; Goldschmidt, E.E. A history of grafting. Hortic. Rev. 2009, 35, 437–494. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Principles of grafting and budding. In Plant Propagation. Principles and Practices, 8th ed.; Pearson: London, UK, 2010; Chapter 11; ISBN 978-0-13-501449-3. [Google Scholar]
- Hartman, G.; Pawlowski, M.; Herman, T.; Eastburn, D. Organically Grown Soybean Production in the USA: Constraints and Management of Pathogens and Insect Pests. Agronomy 2016, 6, 16. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Fiore, A.; Montemurro, F.; Canali, S. Agro-Ecology for Potential Adaptation of Horticultural Systems to Climate Change: Agronomic and Energetic Performance Evaluation. Agronomy 2017, 7, 35. [Google Scholar] [CrossRef]
- Pradeep, K.; Youssef, R.; Mariateresa, C.; Giuseppe, C. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar]
- Moore, R. A model for graft compatibility-incompatibility in higher plants. Am. J. Bot. 1984, 71, 751–758. [Google Scholar] [CrossRef]
- De Miguel, A.; Cebolla, V. Terralia 53, Pages 50–60. Octubre 2005. Unión del Injerto. Available online: https://www.terralia.com/terralias/view_report?magazine_report_id=365 (accessed on 1 September 2018).
- Acosta Muñoz, A. La Técnica del Injerto en Plantas Hortícolas. Horticom (Extra Viveros I), Extra 2005. pp. 62–65. Available online: http://bit.do/eAgQc (accessed on 15 September 2018).
- Saravana Kumar, R.M.; Gao, L.X.; Yuan, H.W.; Xu, D.B.; Liang, Z.; Tao, S.C.; Guo, W.B.; Yan, D.L.; Zheng, B.S.; Edqvist, J. Auxin enhances grafting success in Carya cathayensis (Chinese hickory). Planta 2018, 247, 761–772. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- De Velasco Alvarado, M.J. Tesis: Anatomía y Manejo Agronómico de Plantas Injertadas en Jitomate. Universidad Autónoma de Chapingo (Mexico), 2013. Available online: https://chapingo.mx/horticultura/pdf/tesis/TESISMCH2013050810128186.pdf (accessed on 15 September 2018).
- Leonardi, C.; Romano, D. Recent issues on vegetable grafting. Acta Hortic. 2004, 631, 163–174. [Google Scholar] [CrossRef]
- Yang, S.; Xiang, G.; Zhang, S.; Lou, C. Electrical resistance as a measure of graft union. J. Plant Physiol. 1993, 141, 98–104. [Google Scholar] [CrossRef]
- Bletsos, F.A.; Olympios, C.M. Rootstocks and Grafting of Tomatoes, Peppers and Eggplants for Soil-borne Disease Resistance, Improved Yield and Quality. 2008. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0812/EJPSB_2(SI1)/EJPSB_2(SI1)62-73o.pdf (accessed on 15 September 2018).
- Torii, T.; Kasiwazaki, M.; Okamoto, T.; Kitani, O. Evaluation of graft-take using a thermal camera. Acta Hortic. 1992, 319, 631–634. [Google Scholar] [CrossRef]
- Oda, M.; Maruyama, M.; Mori, G. Water Transfer at Graft Union of Tomato Plants Grafted onto Solanum Rootstocks. J. Jpn. Soc. Hortic. Sci. 2005, 74, 458–463. [Google Scholar] [CrossRef]
- De Miguel, A.; Cebolla, V. Terralia 53, Pages 50–60. Octubre 2005; Unión del Injerto. [Google Scholar]
- Bausher, M.G. Road, South Rock, Pierce, Fort Graft Angle and Its Relationship to Tomato Plant Survival. Hort Sci. 2013, 48, 34–36. Available online: http://hortsci.ashspublications.org/content/48/1/34.short (accessed on 15 September 2018).
- Turquois, N.; Malone, M. Non-destructive assessment of developing hydraulic connections in the graft union of tomato. J. Exp. Bot. 1996, 47, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Sarafis, V.; Campbell, E.O.; Callaghan, P.T. Non invasive imaging of water flow in plants by NMR microscopy. Protoplasma 1993, 173, 170–176. [Google Scholar] [CrossRef]
- Mutisya, S.; Saidi, M.; Opiyo, A.; Ngouajio, M.; Martin, T. Synergistic Effects of Agronet Covers and Companion Cropping on Reducing Whitefly Infestation and Improving Yield of Open Field-Grown Tomatoes. Agronomy 2016, 6, 42. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Tian, S.; Xu, D. Current status of grafting robot for vegetable. In Proceedings of the 2011 International Conference on Electronic & Mechanical Engineering and Information Technology, Harbin, China, 12–14 August 2011; Volume 4, pp. 1954–1957. [Google Scholar] [CrossRef]
- Ito, T. Present state of transplant production practices in Japanese horticultural industry. In Proceedings of the International Symposium on Transplant Production Systems, Transplantant Production Systems, Yokohama, Japan, 21–26 July 1992; Kurata, K., Kozai, T., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1992. Available online: http://0-www-springer-com.brum.beds.ac.uk/us/book/9780792317975 (accessed on 15 September 2018).
- Lee, J.-M.; Oda, M. Grafting of Herbaceous Vegetable and Ornamental Crops. Hortic. Rev. 2003, 28. [Google Scholar] [CrossRef]
- Nishiura, Y.; Murase, H.; Honami, N.; Taira, T. Devlopment of Plug-in Grafting Robotic System Osaka Prefecture University. In Proceedings of the IEEE Lnternatlonal Conference on Robotics and Automatlon, Nagoya, Japan, 25–27 May 1995; pp. 2510–2517. Available online: http://0-ieeexplore-ieee-org.brum.beds.ac.uk/abstract/document/525636/ (accessed on 15 September 2018).
- Oda, M. Grafting of Vegetable Crops. Osaka Perfecture Univ. 2002, 54, 49–72. Available online: http://repository.osakafu-u.ac.jp/dspace/bitstream/10466/1053/1/KJ00000052064.pdf (accessed on 15 September 2018).
- Oda, M. Use of Grafted Seedlings for Vegetable Production in Japan. Acta Hortic. 2008, 770, 15–20. Available online: https://www.actahort.org/books/770/770_1.htm (accessed on 15 September 2018). [CrossRef]
- Lee, J.-M.; Bang, H.J.; Ham, H.S. Grafting of Vegetables. Jpn. Soc. Agric. Mach. Food Eng. 1998, 67, 1098–1104. Available online: https://www.jstage.jst.go.jp/article/jjshs1925/67/6/67_6_1098/_pdf (accessed on 15 September 2018). [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato Grafting: A Global Perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef]
- Same as 49 Miles, C.; Flores, M.; Estrada, E. Hoja de Datos de la Extensión, FS052ES. INJERTO de Verduras Berenjenas y Tomates; Washington State University: Washington, DC, USA, 2013; pp. 1–4. Available online: http://cru.cahe.wsu.edu/CEPublications/FS052ES/FS052ES.pdf (accessed on 15 September 2018).
- Same as 52 De Miguel Gómez, A. Serie Documentos: El Injerto de Plantas de Tomate. Postcosecha 2011, Www.poscosecha.com–Postharvest, Www.postharvest.biz. Available online: www.poscosecha.com/es/publicaciones/ (accessed on 15 September 2018).
- Alvarez, C.; Herzog, D. La técnica del injerto (Presentation by Rijk Zwaan Ibérica). Rijk Zwaan Ibérica, 2011. Available online: https://es.slideshare.net/directorarica/presentacin-injerto-semilleros (accessed on 15 September 2018).
- Oda, M. Grafting of Vegetables to Improve Greenhouse Production; Food & Fertilizer Technology Center: Kawana, Japan, 1998; pp. 1–11. Available online: http://www.agnet.org/library.php?func=view&id=20110803135029 (accessed on 15 September 2018).
- Villasana Rojas, J.A. Tesis: Efecto del Injerto en la Producción de Tomate (Lycopersicon esculentum Mill.) Bajo Condiciones de Invernadero en Nuevo León; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, Mexico, 2010; Available online: http://eprints.uanl.mx/5613/1/1080194762%20%281%29.PDF (accessed on 15 September 2018).
- Yamada, H. Research for Development of the Grafting Robot for Solanaceae. Tech. Pap. Agric. Mach. Res. Assoc. 2003, 65, 142–149. Available online: https://www.jstage.jst.go.jp/article/jsam1937/65/5/65_5_142/_pdf/-char/ja (accessed on 15 September 2018).
- Oda, M.; Tsuji, K.; Sasaki, H. Effect of Hypocotyl Morphology on Survival Rate and Growth of Cucumber Seedling Grafted on Cucurbita ssp. Japan Agricultural Research Quarterly. 1993. Available online: http://www.jircas.affrc.go.jp/english/publication/jarq/26-4/26-4-259-263.pdf (accessed on 15 September 2018).
- Same as 46 Oda, M. Vegetable seedling grafting in Japan. Acta Hortic. 2007, 759, 175–180. [Google Scholar]
- Ashraf, M.A.; Kondo, N.; Shiigi, T. Use of Machine Vision to Sort Tomato Seedlings for Grafting Robot. Eng. Agric. Environ. Food 2011, 4, 119–125. [Google Scholar] [CrossRef]
- Tian, S.; Ashraf, M.A.; Kondo, N.; Shiigi, T.; Momin, M.A. Optimization of Machine Vision for Tomato Grafting Robot. Sens. Lett. 2013, 11, 1190–1194. [Google Scholar] [CrossRef]
- Division, P.B.; Crops, O. Use of Rootstocks in Solanaceous Fruit- Vegetable Production in Japan Plant Breed; JIRCIS: Tsukuba, Japan, 1980. [Google Scholar]
- Camacho Ferre, F.; (Coordinador Obra). V. autores. Técnicas de Producción de Cultivos Protegidos. Tomo II. Caja Rural Intermediterránea, Instituto Cajamar. Ediciones Agrotécnicas, S.L.; II, 776. 2003. Available online: http://www.publicacionescajamar.es/pdf/series-tematicas/agricultura/tecnicas-de-produccion-en-cultivos.pdf (accessed on 15 September 2018).
- Gaion, L.A.; Braz, L.T.; Carvalho, R.F. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2017, 1–18. [Google Scholar] [CrossRef]
- Montsanto, De Ruiter Product guide. Rut. Prod. Guid. 2012. Available online: http://www.deruiterseedstemp.com/global/au/products/Documents/De Ruiter AU Product Guide.pdf (accessed on 15 September 2018).
- Johnson, S.; Kreider, P.; Miles, C. Vegetable Grafting: Eggplants and Tomatoes. 2013. Available online: http://extension.wsu.edu/publications/wp-content/uploads/sites/54/publications/fs052e.pdf (accessed on 15 September 2018). Washington State Univ.; WSU Mount Vernon Northwestern Washington, Fact Sheet, 1–6.
- Rivard, C.L.; Louws, F.J. Grafting for Disease Resistance in Heirloom Tomatoes; AG-675; North Carolina Cooperative Extension Service: Raleigh, NC, USA, 2007; pp. 1–8. [Google Scholar]
- Bumgarner, N.R.; Kleinhenz, M.D. Grafting Guide: A Pictorial Guide to the Cleft and Splice Graft Methods as Applied to Tomato and Pepper. Ohio State University. Research and Development Center, 2014. Available online: http://web.extension.illinois.edu/smallfarm/downloads/50570.pdf (accessed on 15 September 2018).
- Oda, M. New Grafting Methods for Fruit-Bearing Vegetables in Japan. Jpn. Agric. Res. Q. 1995, 194, 187–194. [Google Scholar]
- Maurya, A.K. Role of Grafting in vegetable Production. Vegetable Grafting. GB Pant University of Agriculture and Technology, Pantnagar Uttarakhand, 2014. Available online: http://es.slideshare.net/ashish7891/vegetable-grafting (accessed on 15 September 2018).
- Oda, M. Grafting of Vegetables to Improve Greenhouse Production, p. 1–11. Extension Bulletin—Food & Fertilizer Technology Center, 1999. Available online: https://es.scribd.com/document/220401141/Veggie-Grafting (accessed on 15 September 2018).
- Torres, A.P.; Lopez, G.R. Medición de Luz Diaria Integrada en Invernaderos. Purdue Agriculture. Available online: https://www.extension.purdue.edu/extmedia/HO/HO-238-SW.pdf (accessed on 15 September 2018).
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable grafting: History, use, and current technology status in North America. Hortscience 2008, 43, 1664–1669. [Google Scholar]
- Velasco-alvarado, M.D.J.; Castro-brindis, R.; Avitia-garcía, E.; Castillo-gonzáles, A.M.; Sahagún, J. Proceso de unión del injerto de empalme en jitomate (Solanum lycopersicum L.). Revista Mexicana de Ciencias Agrícolas. 2017. Available online: http://www.redalyc.org/html/2631/263152411004/ (accessed on 15 September 2018).
- Andrews, P.K.; Marquez, C. Graft incompatibility. Hortic. Rev. 1993, 1, 183–232. [Google Scholar]
- De Miguel, Alfredo II Jornadas Sobre Semillas y Semilleros Hortícolas. Libro Editado: ISBN: 84-87564-40-2 1997, 216. Available online: http://www.juntadeandalucia.es/export/drupaljda/1337170141II_Jornadas_sobre_Semillas_y_Semilleros_Horticolas__BAJA.pdf (accessed on 15 September 2018).
- Tateishi, K. Grafting Watermelon onto Pumpkin Suggested Potential Applications. Available online: https://pdfs.semanticscholar.org/2d91/f2d762af27589ecffd858c5eed25bd98ad93.pdf (accessed on 15 September 2018).
- Zhao, X. Grading system of tomato grafting machine based on machine vision. In Proceedings of the 8th International Congress on Image and Signal Processing, Shenyang, China, 14–16 October 2015; pp. 604–609. Available online: http://0-ieeexplore-ieee-org.brum.beds.ac.uk/document/7407950/ (accessed on 15 September 2018).






| Analysis of Variance of Two Factors without Replication | ||||
|---|---|---|---|---|
| Summary | Count | Sum | Mean | Variance |
| 15 to 13 dmm | 10 | 3.3333 | 0.3333 | 0.1806 |
| 13 to 11 dmm | 10 | 3.7242 | 0.3724 | 0.1253 |
| 11 to 9 dmm | 10 | 5.8789 | 0.5879 | 0.1009 |
| 9 to 7 dmm | 10 | 7.1258 | 0.7126 | 0.0589 |
| 7 to 5 dmm | 10 | 8.4487 | 0.8449 | 0.0545 |
| 5 to 3 dmm | 10 | 9.0306 | 0.9031 | 0.0202 |
| 3 to 1 dmm | 10 | 9.1009 | 0.9101 | 0.0245 |
| 1 to (−1) dmm | 10 | 9.2110 | 0.9211 | 0.0284 |
| (−1) to (−3) dmm | 10 | 9.2333 | 0.9233 | 0.0239 |
| (−3) to (−5) dmm | 10 | 8.6000 | 0.8600 | 0.1071 |
| Summary | Count | Sum | Mean | Variance |
| 0° | 10 | 6.7621 | 0.6762 | 0.1398 |
| 10° | 10 | 6.3766 | 0.6377 | 0.1391 |
| 20° | 10 | 6.0410 | 0.6041 | 0.1725 |
| 30° | 10 | 6.9445 | 0.6945 | 0.1644 |
| 40° | 10 | 7.5000 | 0.7500 | 0.1405 |
| 50° | 10 | 8.5650 | 0.8565 | 0.0645 |
| 60° | 10 | 9.5815 | 0.9582 | 0.0031 |
| 70° | 10 | 9.1847 | 0.9185 | 0.0117 |
| 80° | 10 | 9.4521 | 0.9452 | 0.0022 |
| 85° | 10 | 3.2792 | 0.3279 | 0.0326 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Sourface of Variation | Sum of Squares | Degrees of Freedom | Mean of the Squares | F | Probability Value | Critical Value for F |
| Diameter difference | 4.7160 | 9.0000 | 0.5240 | 13.6138 | 0.0000 | 1.9976 |
| Cutting angle | 3.4002 | 9.0000 | 0.3778 | 9.8154 | 0.0000 | 1.9976 |
| Error | 3.1178 | 81.0000 | 0.0385 | |||
| Total | 11.2340 | 99.0000 | ||||
| 0° | 10° | 20° | 30° | 40° | 50° | 60° | 70° | 80° | 85° | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0° | N | N | N | N | Y | Y | Y | Y | Y | |
| 10° | N | N | N | Y | Y | Y | Y | Y | ||
| 20° | N | N | Y | Y | Y | Y | N | |||
| 30° | N | N | N | N | N | Y | ||||
| 40° | N | N | N | N | Y | |||||
| 50° | N | N | N | Y | ||||||
| 60° | N | N | Y | |||||||
| 70° | N | Y | ||||||||
| 80° | Y | |||||||||
| 85° |
| 15 to 13 dmm | 13 to 11 dmm | 11 to 9 dmm | 9 to 7 dmm | 7 to 5 dmm | 5 to 3 dmm | 3 to 1 dmm | 1 to (−1) dmm | (−1) to (−3) dmm | (−3) to (−5) dmm | |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 to 13 dmm | N | Y | Y | Y | Y | Y | Y | Y | Y | |
| 13 to 11 dmm | Y | Y | Y | Y | Y | Y | Y | Y | ||
| 11 to 9 dmm | Y | Y | Y | Y | Y | Y | Y | |||
| 9 to 7 dmm | N | Y | Y | Y | Y | N | ||||
| 7 to 5 dmm | N | N | Y | N | N | |||||
| 5 to 3 dmm | N | N | N | N | ||||||
| 3 to 1 dmm | N | N | N | |||||||
| 1 to (−1) dmm | N | N | ||||||||
| (−1) to (−3) dmm | N | |||||||||
| (−3) to (−5) dmm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Alonso, J.-L.; Carreño-Ortega, Á.; Martínez-Gaitán, C.-C.; Callejón-Ferre, Á.-J. Combined Influence of Cutting Angle and Diameter Differences between Seedlings on the Grafting Success of Tomato Using the Splicing Technique. Agronomy 2019, 9, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9010005
Pardo-Alonso J-L, Carreño-Ortega Á, Martínez-Gaitán C-C, Callejón-Ferre Á-J. Combined Influence of Cutting Angle and Diameter Differences between Seedlings on the Grafting Success of Tomato Using the Splicing Technique. Agronomy. 2019; 9(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9010005
Chicago/Turabian StylePardo-Alonso, José-Luis, Ángel Carreño-Ortega, Carolina-Clara Martínez-Gaitán, and Ángel-Jesús Callejón-Ferre. 2019. "Combined Influence of Cutting Angle and Diameter Differences between Seedlings on the Grafting Success of Tomato Using the Splicing Technique" Agronomy 9, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9010005