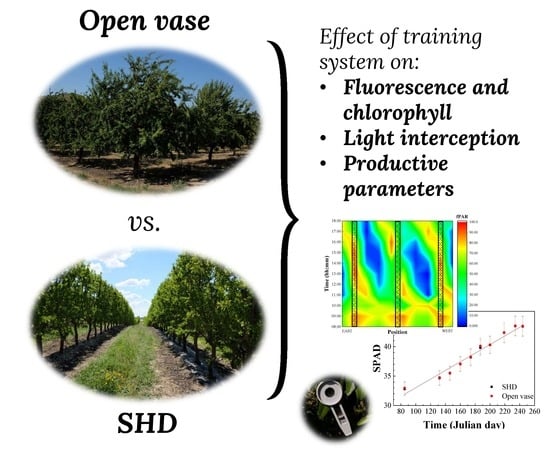
Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards cv. ‘Soleta’
Abstract
:1. Introduction
2. Material and Methods
2.1. Location
2.2. Plant Material and Tree Spacing
2.3. Cultural Practices
2.4. Experimental Design
2.5. Flower Counting, Fruit Counting and Harvest
2.6. Equipment and Measurements
2.7. Statistical Analyses
3. Results
3.1. Fluorescence and Chlorophyll (SPAD) Measurements
3.2. Photosynthetically Active Radiation
3.3. Productive Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Consejería de Agricultura Pesca y Desarrollo Rural. Caracterización del sector de la almendra en Andalucía; Junta de Andalucía: Sevilla, Spain, 2016; p. 37. [Google Scholar]
- MAPA. Estadísticas de superficies y producciones de cultivos. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 10 December 2019).
- Miarnau, X.; Torgueti, L.; Batlle, I.; Romero, A.; Rovira, M.; Alegre, S. La revolución del almendro: Nuevas variedades y modelos productivos. In Proceedings of the Simposio nacional de almendro y otros frutos secos, Lérida, Spain, 24 September 2015; pp. 1–54. [Google Scholar]
- Sansavini, S.; Neri, D.; Tombesi, A.; Continella, G.; Costa, G.; Ramina, A. Impiante e forme di allevamento, potatura, controllo de la fruttificazione e raccolta. In Arboricoltura Generale; Sansavini, S., Costa, G., Gucci, R., Inglese, P., Ramina, A., Xiloyannis, C., Eds.; Patron Editore: Bologna, Italy, 2012; pp. 333–398. [Google Scholar]
- Famiani, P.; Proietti, E.; Lodolini, M.; Neri, D. L’Ulivo e l’Olio, Coltivazione: Gestione della chioma. In Coltura e Cultura; Angelini, R., Ed.; BayerCrop Science S.r.l.: Milano, Italy, 2008; pp. 389–411. [Google Scholar]
- Arquero, O.; Belmonte, A.; Casado, B.; Cruz-Blanco, M.; Espadafor, M.; Fernández, J.; Gallego, J. Consejería de Agricultura, Pesca y Desarrollo Rural, Servicio de Publicaciones y Divulgaciόn. In Manual del almendro; Junta de Andalucía: Sevilla, Spain, 2013; p. 78. [Google Scholar]
- Iglesias, I. Evolución y desarrollo de sistemas de plantación superintensivos en cultivos leñosos. Vida Rural 2019, 472, 50–55. [Google Scholar]
- Iglesias, I. Sistemas de plantación 2D: Una novedad en almendro, una realidad en frutales. Hacia una alta eficiencia. Revista de Fruticultura 2019, 67, 22–44. [Google Scholar]
- Iglesias, I. Costes de producción, sistemas de formación y mecanización en frutales, con especial referencia al melocotonero. Revista de Fruticultura 2019, 69, 50–59. [Google Scholar]
- Roca, J.M.; Gómez, J.M.; López, M. El almendro en seto SHD: La recolección con máquinas cabalgantes. Olint: Revista de plantaciones superintensivas de olivo 2014, 25, 35–47. [Google Scholar]
- Pérez-Ruiz, M.; Rallo, P.; Jiménez, M.; Garrido-Izard, M.; Suárez, M.; Casanova, L.; Valero, C.; Martínez-Guanter, J.; Morales-Sillero, A. Evaluation of Over-The-Row Harvester Damage in a Super-High-Density Olive Orchard Using On-Board Sensing Techniques. Sensors 2018, 18, 1242. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, I.; Whitfield, D.M.; Connor, D.J. Effects of tree size on water use of peach (Prunus persica L. Batsch). Irrig. Sci. 2005, 24, 59–68. [Google Scholar] [CrossRef]
- Williams, L.E.; Ayars, J.E. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agric. For. Meteorol. 2005, 132, 201–211. [Google Scholar] [CrossRef]
- Wagenmakers, P.S.; Callesen, O. Influence of Light Interception on Apple Yield and Fruit Quality Related to Arrangement and Tree Height. Acta Hortic. 1989, 243, 149–158. [Google Scholar] [CrossRef]
- Sander, G.F.; Macedo, T.A.; da Silva, P.S.; Welter, J.F.; Posser, A.J.; Rufato, L.; Kretzschmar, A.A. Effect of different training systems to catch greater light interception in apple cultivar Maxi Gala in temperate climate. Aust. J. Crop Sci. 2019, 13, 574–577. [Google Scholar] [CrossRef]
- Tang, L.; Yin, D.; Chen, C.; Yu, D.; Han, W. Optimal Design of Plant Canopy Based on Light Interception: A Case Study With Loquat. Front. Plant. Sci. 2019, 10, 364. [Google Scholar] [CrossRef]
- Raffo, M.D.; Iglesias, N. Efecto de la intercepción y distribución de la radiación fotosintéticamente activa en manzanos cv. Fuji, bajo cuatro sistemas de conducción en alta densidad. RIA. Revista de Investigaciones Agropecuarias 2004, 33, 29–42. [Google Scholar]
- DeJong, T.; Day, K. Relationships between shoot productivity and leaf characteristics in peach canopies. HortScience 1991, 26, 1271–1273. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, X.; Sinoquet, H.; Vandame, M. Spatial distribution of leaf dry weight per area and leaf nitrogen concentration in relation to local radiation regime within an isolated tree crown. Tree Physiol. 1999, 19, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Felipe, A.J. El almendro: El material vegetal; Mira Editores: Zaragoza, Spain, 2000. [Google Scholar]
- Godini, A. Almond Fruitfulness and Role of Self-Fertility. Acta Hortic. 2002, 591, 191–203. [Google Scholar] [CrossRef]
- Kester, D.; Griggs, W. Fruit setting in the almond: The effect of cross-pollinating various percentages of flowers and the pattern of flower and fruit drop. Proc. Am. Soc. Hortic. Sci. 1959, 74, 214–219. [Google Scholar]
- Lampinen, B.; Dejong, T.; Weinbaum, S.; Metcalf, S.; Viveros, M. Spur dynamics and almond productivity. Options Méditerranéennes, Série A 2007, 63, 295–304. [Google Scholar]
- Socias i Company, R.; Felipe, A.J. ‘Belona’ and ‘Soleta’ Almonds. HortScience 2007, 42, 704–706. [Google Scholar] [CrossRef] [Green Version]
- Alonso Segura, J.M.; Socias i Company, R.; Kodad, O.; Espada Carbó, J.L.; Andreu Lahoz, J.; Escartín Santolaria, J. Performance of the CITA almond releases and some elite breeding selections. In Proceedings of the XVI GREMPA Meeting on Almonds and Pistachios, Meknes, Morocco, 12–14 May 2015; pp. 33–36. [Google Scholar]
- Socias i Company, R.; Felipe Mansergas, A. ‘Belona’ y ‘Soleta’, dos nuevos cultivares de almendro. ITEA, información técnica económica agraria: revista de la Asociación Interprofesional para el Desarrollo Agrario 2006, 102, 398–421. [Google Scholar]
- Socias i Company, R.; Alonso, J.M.; Kodad, O. Las heladas y las lluvias: Condicionantes climáticos para el almendro. Agricultura: Revista agropecuaria y ganadera 2009, 921, 626–630. [Google Scholar]
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Valdebenito, D.; Tombesi, S.; Tixier, A.; Lampinen, B.; Brown, P.; Saa, S. Spur behavior in Almond trees (Prunus dulcis [Mill.] DAWebb): Effects of flowers, fruit, and “June drop” on leaf area, leaf nitrogen, spur survival and return bloom. Sci. Hortic. 2017, 215, 15–19. [Google Scholar] [CrossRef]
- Espadafor, M.; Orgaz, F.; Testi, L.; Lorite, I.J.; Villalobos, F.J. Transpiration of young almond trees in relation to intercepted radiation. Irrig. Sci. 2015, 33, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Campillo, C.; Fortes, R.; Henar Prieto, M.D. Solar Radiation Effect on Crop Production. In Solar Radiation; Babatunder, E.B., Ed.; InTechOpen: London, UK, 2012; pp. 167–194. [Google Scholar] [CrossRef] [Green Version]
- Connor, D.J. Towards optimal designs for hedgerow olive orchards. Aust. J. Agric. Res. 2006, 57. [Google Scholar] [CrossRef]
- Iglesias, I.; Alegre, S. The effect of anti-hail nets on fruit protection, radiation, temperature, quality and profitability of ‘Mondial Gala’apples. J. Appl. Hortic. 2006, 8, 91–100. [Google Scholar]
- Núñez, R.; Iglesias i Castellarnau, I.; Montserrat Sangra, R.; Alegre Castellví, S. Eficiencia agronómica de seis sistemas de formación con la variedad de melocotón “Merrill O’Henry” (Prunus persica (Batsch)). ITEA, información técnica económica agraria: revista de la Asociación Interprofesional para el Desarrollo Agrario (AIDA) 2006, 102, 13–26. [Google Scholar]
- Rosati, A. A Simple Method to Estimate Photosynthetic Radiation Use Efficiency of Canopies. Ann. Bot. 2004, 93, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Ranjbarfordoei, A.; Samson, R.; Van Damme, P. Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 2011, 49, 107–111. [Google Scholar] [CrossRef] [Green Version]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Erdal, Í.; Türkmen, R.; Akgün, A. Variations in chlorophyll, SPAD values and some nutrient concentrations depending on chlorosis in peach leaves. Lucrări Ştiinţifice 2016, 59, 13–16. [Google Scholar]
- Ben Yahmed, J.; Ghrab, M.; Ben Mimoun, M. Eco-physiological evaluation of different scion-rootstock combinations of almond grown in Mediterranean conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, S.; Pinochet, J.; Abadía, A.; Moreno, M.Á.; Gogorcena, Y. Tolerance response to iron chlorosis of Prunus selections as rootstocks. HortScience 2008, 43, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Lampinen, B.D.; Udompetaikul, V.; Browne, G.T.; Metcalf, S.G.; Stewart, W.L.; Contador, L.; Negrón, C.; Upadhyaya, S.K. A Mobile Platform for Measuring Canopy Photosynthetically Active Radiation Interception in Orchard Systems. HortTechnology 2012, 22, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Socias i Company, R.; Gradziel, T. Almonds: Botany, Production and Uses; CABI: Boston, MA, USA, 2017. [Google Scholar]
- Kester, D.; Graziel, T. Almonds. In Fruit breeding, Nuts; Janick, J., Moore, J.N., Eds.; Wiley: New York, NY, USA, 1996; Volume 3, pp. 1–98. [Google Scholar]
- Saa, S.; Brown, P.H. Fruit presence negatively affects photosynthesis by reducing leaf nitrogen in almond. Funct. Plant Biol. 2014, 41. [Google Scholar] [CrossRef]
- Tombesi, S.; Lampinen, B.D.; Metcalf, S.; DeJong, T.M. Spur Fruit Set Is Negatively Related with Current-year Spur Leaf Area in Almond. HortScience 2015, 50, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gómez, P.; Prudencio, A.S.; Gradziel, T.M.; Dicenta, F. The delay of flowering time in almond: A review of the combined effect of adaptation, mutation and breeding. Euphytica 2017, 213. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Dorfman, J.; Dorfman, M.; Heien, D. Causes of almond yield variations. Calif. Agric. 1988, 42, 27–28. [Google Scholar]
- Zarate-Valdez, J.L.; Muhammad, S.; Saa, S.; Lampinen, B.D.; Brown, P.H. Light interception, leaf nitrogen and yield prediction in almonds: A case study. Eur. J. Agron. 2015, 66, 1–7. [Google Scholar] [CrossRef]
- Tombesi, S.; Lampinen, B.D.; Metcalf, S.; DeJong, T.M. Yield in almond is related more to the abundance of flowers than the relative number of flowers that set fruit. Calif. Agric. 2017, 71, 68–74. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R. Densidad floral, cuajado y características de los frutos del almendro en relación al tipo de ramificación. ITEA: Información Técnica Económica Agraria 2008, 104, 433–447. [Google Scholar]
- Puebla Arias, M.; Vivas Cacho, A. Resultados de Estudio comparativo de variedades de almendro y nuevos modelos de producción. In Plantaciones en Seto; CICYTEX: Guadajira, Badajoz, 2015; p. 8. [Google Scholar]
- Puebla Arias, M. Estudio comparativo de variedades de almendro y nuevos modelos de producción. In. Plantaciones en Seto; CICYTEX: Guadajira, Badajoz, 2016; p. 10. [Google Scholar]
- Montañés, E. Datos productivos de las fincas pioneras en el almendro en alta densidad. Olint: Revista de plantaciones superintensivas de olivo 2016, 30, 52–55. [Google Scholar]
- Méndez, J. Productividad de nuevas variedades de almendro en el Campo de Cartagena. In Informe Anual de Resultados; CDA Torre-Pachecho-CARM: Torre-Pachecho, Spain, 2018; p. 72. [Google Scholar]
- Lordan, J.; Zazurca, L.; Maldonado, M.; Torguet, L.; Alegre, S.; Miarnau, X. Horticultural performance of ‘Marinada’ and ‘Vairo’ almond cultivars grown on a genetically diverse set of rootstocks. Sci. Hortic. 2019, 256. [Google Scholar] [CrossRef]
- Muhammad, S.; Sanden, B.L.; Saa, S.; Lampinen, B.D.; Smart, D.R.; Shackel, K.A.; DeJong, T.M.; Brown, P.H. Optimization of nitrogen and potassium nutrition to improve yield and yield parameters of irrigated almond (Prunus dulcis (Mill.) D. A. webb). Sci. Hortic. 2018, 228, 204–212. [Google Scholar] [CrossRef]
- Iglesias, I. El almendro en seto autoenraizado: Una nueva alternativa para los secanos. Horticultura 2019, 344, 24–33. [Google Scholar]
- Duncan, R. Choosing the Correct Tree Spacing for Your Almond Orchard. Available online: http://cestanislaus.ucanr.edu/files/111776.pdf (accessed on 12 November 2019).






| Parameter | Super High Density (SHD) | Open-Center | ||
|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | |
| pH (ext. 1:2.5 H2O) | 7.97 | 8.02 | 7.90 | 7.83 |
| EC 25 °C (ext. 1:5 H2O), dS/m | 2.49 | 2.40 | 2.61 | 2.51 |
| OM (W&B), % | 1.92 | 1.13 | 1.42 | 1.28 |
| CaCO3 eq., % | 28 | 29 | 29 | 32 |
| N-NO3, mg/kg | 172 | 164.9 | 95.3 | 71.5 |
| P (Olsen), mg/kg | 23 | <5.0 | 11.3 | 5.5 |
| K (ammonium acetate ext.), mg/kg | 256 | 96 | 334 | 125 |
| Ca (ammonium acetate ext.), mg/kg | 9090 | 9619 | 3325,2 | 3851,9 |
| Particle-size class (USDA) | Silty clay loam | Silty clay loam | Silt loam | Silt loam |
| Active lime, % | 7 | 7 | 6 | 6 |
| Parameter | 2018 | 2019 | Kruskal–Wallis | ||
|---|---|---|---|---|---|
| SHD | Open-Center | SHD | Open-Center | ||
| No. flowers/tree | 2134 ± 1120 b | 26918 ± 6949 a | 703 ± 920 c | 10608 ± 3723 ab | <0.0001 |
| No. set fruits/tree | 415 ± 90 b | 4078 ± 1068 a | 323 ± 226 c | 2286 ± 502 ab | 0.000 |
| Fruit set rate (%) | 22.8 ± 10.3 b | 20.9 ± 5.6 ab | 42.0 ± 21.8 a | 21.5 ± 3.1 ab | 0.016 |
| Almond fresh fruit yield (kg/tree) | 3.30 ± 0.91 b | 78.82 ± 22.03 a | 3.00 ± 1.73 b | 53.00 ± 8.00 a | 0.001 |
| Almond in shell yield (kg/tree) | 1.77 ± 0.51 b | 34.66 ± 4.85 a | 1.69 ± 1.22 b | 24.92 ± 7.64 a | 0.002 |
| Kernel yield (kg/tree) | 0.63 ± 0.06 c | 10.25 ± 1.58 a | 0.53 ± 0.42 c | 6.19 ± 1.82 b | 0.001 |
| Almond in shell yield (kg/ha) * | 4425 | 9627 | 4225 | 6922 | |
| Kernel yield (kg/ha) * | 1575 | 2847 | 1325 | 1719 | |
| Almond in shell weight (g) | 2.38 ± 0.16 b | 3.32 ± 0.19 a | 3.53 ± 1.09 a | 4.10 ± 0.25 a | <0.0001 |
| Kernel weight (g) | 0.93 ± 0.03 b | 1.18 ± 0.08 a | 1.11 ± 0.30 a | 1.12 ± 0.08 a | <0.0001 |
| Kernel yield (%) | 38.33 ± 1.61 a | 35.66 ± 0.40 b | 31.45 ± 1.39 c | 29.52 ± 1.27 d | <0.0001 |
| Canopy volume (m3) | 2.05 d | 59.80 ± 1.25 a | 2.25 c | 58.40 ± 1.70 b | <0.0001 |
| Yield per canopy volume (g kernel/m3) | 306.9 ± 31.6 a | 171.4 ± 23.2 b | 233.9 ± 185.3 ab | 106.0 ± 34.2 b | <0.0001 |
| TCSA (cm2) | 56.7 ± 15.7 b | 344.4 ± 54.6 a | 62.4 ± 16.0 b | 368.0 ± 63.0 a | 0.001 |
| Yield efficiency (g kernel/cm2) | 11.52 ± 1.80 b | 26.79 ± 2.69 a | 9.07 ± 6.56 c | 18.10 ± 5.62 ab | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Gascón, J.; Figueras-Panillo, M.; Iglesias-Castellarnau, I.; Martín-Ramos, P. Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards cv. ‘Soleta’. Agronomy 2019, 9, 874. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120874
Casanova-Gascón J, Figueras-Panillo M, Iglesias-Castellarnau I, Martín-Ramos P. Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards cv. ‘Soleta’. Agronomy. 2019; 9(12):874. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120874
Chicago/Turabian StyleCasanova-Gascón, José, Marcos Figueras-Panillo, Ignasi Iglesias-Castellarnau, and Pablo Martín-Ramos. 2019. "Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards cv. ‘Soleta’" Agronomy 9, no. 12: 874. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120874