Cladobotryum mycophilum as Potential Biocontrol Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Growth Conditions of the Cladobotryum Isolates in PDA
2.3. Screening Test of Antagonistic Isolates
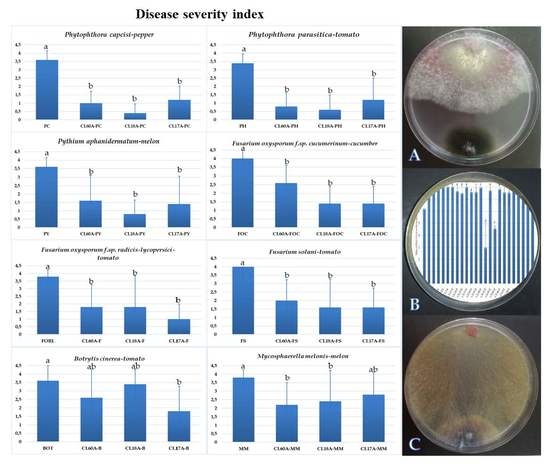
2.4. Greenhouse Evaluation of Selected Antagonists of C. mycophilum on the Severity of Eight Phytopathogens
2.5. Statistical Analysis
3. Results
3.1. Colony Growth of Cladobotryum Isolates
3.2. Effects of Cladobotryum Isolates on the Radial Growth of Phytopathogens
3.3. Biological Control of Cladobotryum Isolates against Several Diseases
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control; Manson Publishing: London, UK, 2008. [Google Scholar]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Incidence, identification and pathogenicity of Cladobotryum mycophilum, causal agent of cobweb disease on Agaricus bisporus mushroom crops in Spain. Ann. Appl. Biol. 2016, 168, 214–224. [Google Scholar] [CrossRef]
- Chakwiya, A.; Van der Linde, E.J.; Chidamba, L.; Korsten, L. Diversity of Cladobotryum mycophilum isolates associated with cobweb disease of Agaricus bisporus in the south African mushroom industry. Eur J. Plant. Pathol. 2019, 154, 767–776. [Google Scholar] [CrossRef]
- Adie, B.A.T. The Biology and Epidemiology of the Cobweb Disease Pathogen (Cladobotryum Spp.) Infecting the Cultivated Mushroom (Agaricus Bisporus). Ph.D. Thesis, Imperial College, University of London, London, UK, 2000. [Google Scholar]
- Carrasco, J.; Navarro, M.J.; Gea, F.J. Cobweb, a serious pathology in mushroom crops: A review. Span. J. Agric. Res. 2017, 15, e10R01. [Google Scholar] [CrossRef] [Green Version]
- Gea, F.J.; Navarro, M.J.; Suz, L.M. First report of Cladobotryum mycophilum causing cobweb on cultivated king oyster mushroom in Spain. Plant. Dis. 2011, 95, 1030. [Google Scholar] [CrossRef] [PubMed]
- Back, C.G.; Lee, C.Y.; Seo, G.S.; Jung, H.Y. Characterization of species of Cladobotryum which cause cobweb disease in edible mushrooms grown in Korea. Mycobiology 2012, 40, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Lee, Y.H.; Cho, K.M.; Lee, J.Y. First report of cobweb disease caused by Cladobotryum mycophilum on the edible mushroom Pleurotus eryngii in Korea. Plant. Dis. 2012, 96, 1374. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Suz, L.M. Cobweb disease on oyster culinary medicinal mushroom (Pleurotus ostreatus) caused by the mycoparasite Cladobotryum mycophilum. J. Plant. Pathol. 2019, 101, 349–354. [Google Scholar] [CrossRef]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Gea, F.J. Effect of five fungicides with different modes of action on mushroom cobweb disease (Cladobotryum mycophilum) and mushroom yield. Ann. Appl. Biol. 2017, 171, 62–69. [Google Scholar] [CrossRef]
- Barnett, H.L.; Binder, F.L. The fungal host-parasite relationship. Annu. Rev. Phytopathol. 1973, 11, 273–292. [Google Scholar] [CrossRef]
- Rudakov, O.L. Physiological groups in mycophilic fungi. Mycologia 1978, 70, 150–159. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild predation among biological-control agents—Theoryand evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Bastos, C.N.; Evans, H.C.; Samson, R.A. A new hyperparasitic fungus, Cladobotryum amazonense, with potential for control of fungal pathogens of cocoa. Trans. Br. Mycol. Soc. 1981, 77, 273–278. [Google Scholar] [CrossRef]
- Marzuki, N.F.; Goh, Y.K.; Tung, H.J.; Goh, Y.K.; Goh, K.J. Evaluation on the cultural characteristics and antagonistic activities of Cladobotryum semicirculare against Ganoderma boninense in vitro. J. Oil Palm Res. 2015, 27, 326–338. [Google Scholar]
- Ramos, B. Actividad fungicida de cepas de Cladobotryum spp., para el control de hongos patógenos presentes en modalidades productivas de la agricultura urbana. Agrotec. Cuba 2018, 42, 98–99. [Google Scholar]
- Santos, M.; Diánez, F.; González, M.; Tello, J.C. Grape marc compost: Microbial studies and suppression of soilborne mycosis in vegetable seedlings. World J. Microbiol. Biotechnol. 2008, 24, 1493–1505. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Carretero, F.; Marín, F. Trichoderma saturnisporum, a new biological control agent. J. Sci. Food Agric. 2016, 96, 1934–1944. [Google Scholar] [CrossRef]
- Siozios, S.; Tosi, L.; Ferrarini, A.; Ferrari, A.; Tononi, P.; Bellin, D.; Maurhofer, M.; Gessler, C.; Delledonne, M.; Pertot, I. Transcriptional reprogramming of the mycoparasitic fungus Ampelomyces quisqualis during the powdery mildew host induced germination. Phytopathology 2015, 105, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.C.; Pfender, W.F. Effects of the Mycoparasite Sphaerellopsis filum on Overwintering Survival of Stem Rust in Perennial Ryegrass. Plant. Dis. 2012, 96, 1471–1481. [Google Scholar] [CrossRef] [Green Version]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system—A review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its multifarious utility in crop improvement. In Crop Improvement through Microbial Biotechnology. New and Future Developments in Microbial Biotechnology and Bioengineering; Aligarh Muslim University: Aligarh, India; Srinagar, India, 2018; Chapter 13; pp. 263–291. [Google Scholar]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, S.; Bordner, J.; Decosta, D.L.; Dekker, K.A.; Hirai, H.; Inagaki, T.; Kim, Y.; Sugiura, A.; Sutcliffe, J.A.; Tachikawa, K.; et al. CJ-15,696 and its analogs, new furopyridine antibiotics from the fungus Cladobotryum varium: Fermentation, isolation, structural elucidation, biotransformation and antibacterial activities. J. Antibiot. 2002, 55, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Blunt, J.W.; Cole, A.L.; Cannon, J.F.; Robinson, W.T.; Munro, M.H. Two novel cytotoxic cyclodepsipeptides from a mycoparasitic Cladobotryum sp. J. Org. Chem. 2003, 68, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Lang, G.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.; Robinson, W.T.; Munro, M.H. Cladobotric acids AF: New cytotoxic polyketides from a New Zealand Cladobotryum sp. J. Org. Chem. 2006, 71, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, J.; Jensen, H.C.; Kjaer, A.; Olsen, C.E.; Rassing, B.R.; Søtofte, I. Cladobotryal: A fungal metabolite with a novel ring system. Acta Chem. Scand. 1998, 52, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.N. Effect of the culture filtrate of Cladobotryum amazonense on Crinipellis perniciosa (Stahel) Singer and other pathogens. Rev. Theobroma 1984, 14, 263–269. [Google Scholar]
- Goh, Y.K.; Marzuki, Y.N.; Liew, F.A.; Goh, Y.K.; Goh, K.J. Antagonistic effects of fungicolous ascomycetous Cladobotryum Semicirculare on Rigidoporus Microporus White Root Disease in Rubber Trees (Hevea Brasiliensis) under in vitro and Nursery Experiments. J. Rubber Res. 2018, 21, 62–72. [Google Scholar] [CrossRef]



| Isolates | % Inhibition of Mycelial Growth | |||||||
|---|---|---|---|---|---|---|---|---|
| Plant Pathogens | ||||||||
| Botrytis cinerea | FORL | Fusarium solani | FOC | Phytophthora parasitica | Mycosphaerella melonis | Phytophthora capsici | Pythium aphanidermatum | |
| CLR1A | 68.25 ± 1.89 cdefg | 72 ± 2.44 bc | 76 ± 2.05 cdefg | 73.75 ± 3.19 abc | 81.25 ± 10.72 cdefg | 86.5 ± 7.68 ab | 66.5 ± 15.72 efg | 49.25 ± 1.42 defg |
| CLR2A | 68.5 ± 1.63 cdefg | 71.25 ± 3.75 bc | 77.5 ± 0.88 cdef | 72.75 ± 2.05 abc | 89.25 ± 0.68 abcdef | 80.5 ± 3.38 abc | 77 ± 2.88 abcd | 48 ± 4.29 efghi |
| CLR2B | 68.75 ± 3.53 cdefg | 71.5 ± 1.05 bc | 79.25 ± 1.12 bcd | 72.25 ± 2.40 abc | 89.5 ± 2.09 abcde | 72 ± 1.68 cd | 74 ± 1.85 bcde | 51.5 ± 2.05 cdef |
| CL5A | 58.75 ± 12.37 hi | 47.75 ± 6.09 ef | 70.25 ± 6.52 h | 46.5 ± 7.97 g | 71.75 ± 11.20 h | 60.62 ± 11.39 e | 63 ± 14.19 fgh | 45.75 ± 3.25 ghijk |
| CL6A | 73.5 ± 3.23 abc | 76.25 ± 2.34 ab | 82.5 ± 2.17 ab | 78.25 ± 4.20 a | 91 ± 2.85 ab | 79.25 ± 3.26 abc | 77.25 ± 1.63 abcd | 53.25 ± 5.19 bcde |
| CL7A | 76.25 ± 1.97 ab | 73.25 ± 1.12 ab | 80 ± 1.25 abc | 76 ± 1.63 ab | 91 ± 1.05 ab | 78.25 ± 4.11 bc | 79.75 ± 3.24 ab | 53.5 ± 2.40 bcd |
| CL11A | 74.5 ± 2.87 abc | 75.75 ± 1.90 ab | 79.75 ± 1.05 abcd | 73 ± 2.44 abc | 92 ± 4.73 ab | 87.5 ± 4.76 ab | 77.75 ± 2.24 abc | 55.25 ± 4.54 abc |
| CL14A | 79.25 ± 12.00 a | 73.75 ± 3.95 ab | 74.75 ± 3.69 efg | 75.75 ± 1.68 ab | 91 ± 2.71 ab | 86.75 ± 1.90 ab | 80.75 ± 1.43 ab | 49 ± 9.28 defg |
| CL15A | 62.75 ± 6.33 gh | 46.75 ± 9.04 ef | 74.75 ± 4.37 efg | 59.75 ± 4.28 f | 80.31 ± 14.10 bcdef | 54 ± 16.52 ef | 65.25 ± 14.43 fg | 43.25 ± 4.72 ijkl |
| CL17A | 73.25 ± 0.68 abc | 80 ± 0.88 a | 83 ± 2.09 ab | 79 ± 0.56 a | 93.75 ± 1.25 a | 85.25 ± 1.37 ab | 83.25 ± 3.60 a | 59.25 ± 2.43 a |
| CL18A | 72.5 ± 1.76 bcd | 76.5 ± 1.63 ab | 79.5 ± 4.11 abcd | 76.25 ± 1.98 a | 91.75 ± 0.68 ab | 85.5 ± 2.59 ab | 81.25 ± 1.53 ab | 58.25 ± 1.42 ab |
| CL19A | 64.25 ± 1.67 fgh | 32.18 ± 2.13 h | 74.5 ± 2.88 efg | 30.83 ± 1.91 h | 72.5 ± 5.00 gh | 38.25 ± 2.44 g | 55.25 ± 10.73 h | 36.25 ± 8.14 m |
| CL40A | 71.5 ± 1.04 bcde | 48.5 ± 2.40 ef | 79.75 ± 2.24 abcd | 75.25 ± 1.63 ab | 90 ± 3.75 abcd | 62.5 ± 5.80 de | 74.25 ± 1.12 bcde | 48.5 ± 2.40 defghi |
| CL41A | 47.25 ± 10.24 j | 42 ± 12.39 fg | 72 ± 3.60 gh | 32.75 ± 1.63 h | 66.67 ± 18.76 fgh | 46.75 ± 14.16 fg | 62.75 ± 8.45 fgh | 21 ± 1.62 n |
| CL43A | 70 ± 2.16 bcdef | 61.25 ± 18.54 d | 79 ± 3.58 bcd | 60 ± 20.54 ef | 92 ± 2.27 ab | 80.25 ± 3.79 abc | 76 ± 6.93 abcd | 53.5 ± 7.72 bcd |
| CL45A | 53 ± 5.49 ij | 51 ± 3.79 e | 70.25 ± 2.71 h | 67 ± 6.47 cde | 80.75 ± 5.90 defgh | 64 ± 5.89 de | 67 ± 3.81 efg | 38.5 ± 4.08 lm |
| CL46A | 65.75 ± 3.37 efg | 53.5 ± 9.82 e | 76.5 ± 5.89 cdef | 64 ± 2.85 def | 86.25 ± 9.80 abcdef | 54.75 ± 10.66 ef | 60 ± 11.28 gh | 40.5 ± 4.80 klm |
| CL2A | 71.5 ± 3.46 bcde | 72 ± 1.43 bc | 77.75 ± 1.37 cdef | 74 ± 1.85 ab | 90.75 ± 2.74 ab | 88.75 ± 4.59 a | 79.25 ± 3.49 abc | 48.75 ± 4.05 defgh |
| CL30A | 68 ± 1.89 cdefg | 75 ± 1.25 ab | 79.25 ± 1.43 bcd | 75.5 ± 2.09 ab | 90.5 ± 0.68 abc | 82 ± 3.38 abc | 78.75 ± 1.53 abc | 51.5 ± 1.04 cdef |
| CL55A | 70.5 ± 8.50 bcdef | 70.25 ± 2.85 bc | 78.25 ± 6.29 cde | 72.75 ± 3.35 abc | 87.75 ± 1.05 abcdef | 77.75 ± 3.99 bc | 76 ± 2.05 abcd | 47 ± 3.81 fghij |
| CL60A | 76.25 ± 1.97 ab | 76.5 ± 3.47 ab | 83.5 ± 4.18 a | 76.75 ± 1.12 a | 91.5 ± 1.85 ab | 86.75 ± 1.90 ab | 80 ± 2.34 ab | 57.75 ± 1.85 ab |
| CL80A | 68.75 ± 4.33 cdefg | 35.5 ± 5.20 gh | 75.75 ± 1.43 defg | 69 ± 6.34 bcd | 88.75 ± 11.39 abcdef | 61 ± 22.73 e | 71 ± 1.63 cdef | 46.25 ± 4.23 fghij |
| CL1264 | 70.25 ± 1.85 bcdef | 74 ± 1.85 ab | 73.75 ± 4.15 fgh | 75.25 ± 1.37 ab | 93.25 ± 2.44 ab | 80.75 ± 3.71 abc | 77 ± 1.90 abcd | 42.25 ± 4.08 jkl |
| CL1381 | 66.25 ± 1.25 defg | 65 ± 2.05 cd | 74.75 ± 1.05 efg | 46.75 ± 2.05 g | 80.25 ± 3.47 efgh | 62.25 ± 2.05 de | 69 ± 2.05 def | 43.5 ± 2.23 hijkl |
| p | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.; Diánez, F.; Moreno-Gavíra, A.; Sánchez-Montesinos, B.; Gea, F.J. Cladobotryum mycophilum as Potential Biocontrol Agent. Agronomy 2019, 9, 891. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120891
Santos M, Diánez F, Moreno-Gavíra A, Sánchez-Montesinos B, Gea FJ. Cladobotryum mycophilum as Potential Biocontrol Agent. Agronomy. 2019; 9(12):891. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120891
Chicago/Turabian StyleSantos, Mila, Fernando Diánez, Alejandro Moreno-Gavíra, Brenda Sánchez-Montesinos, and Francisco J. Gea. 2019. "Cladobotryum mycophilum as Potential Biocontrol Agent" Agronomy 9, no. 12: 891. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9120891