Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pepper Crops
2.3. Plant Material
2.4. Fertilization
2.5. Monitoring of the Fruit Ripening and Pepper Harvesting
2.6. Ultrasound Assisted Extraction of Capsaicinoids
2.7. Determination of Total and Individual Capsaicinoids
2.7.1. UHPLC-Fluorescence Analysis
2.7.2. UHPLC-Q-ToF-MS Analysis
2.8. Statistical Analysis
3. Results and Discussion
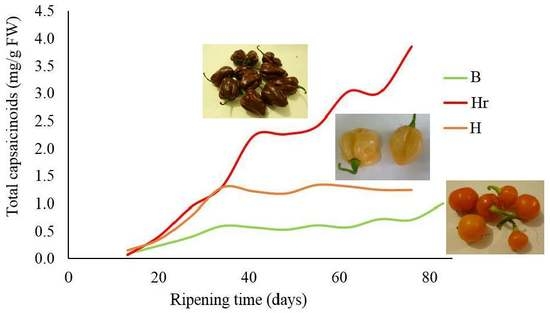
3.1. Evolution of the Total Capsaicinoids Content in Capsicum chinense Cultivars
3.2. Evolution of the Individual Contents of Capsaicinoids in Capsicum chinense Cultivars
3.3. Evolution of the Relative Percentage of Capsaicinoids in Capsicum chinense Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. Bioactive compounds of four hot pepper varieties (Capsicum annuum L.), antioxidant capacity, and intestinal bioaccessibility. J. Agric. Food Chem. 2010, 58, 3399–3406. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Variation of antioxidant activity and the levels of bioactive compounds in lipophilic and hydrophilic extracts from hot pepper (Capsicum spp.) cultivars. Food Chem. 2012, 134, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; De Luca, D.; de Cindio, B.; Menichini, F. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (Acuminatum small and Cerasiferum). Plant Food Hum. Nutr. 2011, 66, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Thán, M.; Németh, J.; Szilvássy, Z.; Pintér, E.; Helyes, Z.; Szolcsányi, J. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur. J. Pharmacol. 2000, 399, 251–258. [Google Scholar] [CrossRef]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A Comprehensive Review of the Carcinogenic and Anticarcinogenic Potential of Capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From plants to a cancer-suppressing agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Brooks, J.R. Capsaicin-Based Therapies for Pain Control. In Capsaicin as a Therapeutic Molecule; Abdel-Salam, O.M.E., Ed.; Springer: Basel, Switzerland, 2014; pp. 129–146. [Google Scholar] [CrossRef]
- Joo, J.I.; Kim, D.H.; Choi, J.-W.; Yun, J.W. Proteomic Analysis for Antiobesity Potential of Capsaicin on White Adipose Tissue in Rats Fed with a High Fat Diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef]
- Wall, M.M.; Waddell, C.A.; Bosland, P.W. Variation in β-Carotene and Total Carotenoid Content in Fruits of Capsicum. HortScience 2001, 36, 746. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwai, K. Chapter 4 Constituents of Red Pepper Species: Chemistry, Biochemistry, Pharmacology, and food Science of the Pungent Principle of Capsicum Species. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press: Orlando, FL, USA, 1984; Volume 23, pp. 227–299. [Google Scholar]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Momin, C.M.; Acharya, P.; Kabir, J.; Debnath, M.K.; Dhua, R.S. Dynamics of changes in bioactive molecules and antioxidant potential of Capsicum chinense Jacq. cv. Habanero at nine maturity stages. Acta Physiol. Plant. 2013, 35, 1141–1148. [Google Scholar] [CrossRef]
- Bennett, D.J.; Kirby, G.W. Constitution and biosynthesis of capsaicin. J. Chem. Soc. (C) 1968, 442–446. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharma, S.S.; Sinha, P.; Negi, M.S.; Neog, B.; Tripathi, S.B. Variability in capsaicinoid content in different landraces of Capsicum cultivated in north-eastern India. Sci. Hortic. 2015, 183, 66–71. [Google Scholar] [CrossRef]
- Baas-Espinola, F.M.; Castro-Concha, L.A.; Vázquez-Flota, F.A.; Miranda-Ham, M.L. Capsaicin synthesis requires in situ phenylalanine and valine formation in in vitro Maintained Placentas from Capsicum chinense. Molecules 2016, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Perucka, I.; Oleszek, W. Extraction and determination of capsaicinoids in fruit of hot pepper Capsicum annuum L. by spectrophotometry and high-performance liquid chromatography. Food Chem. 2000, 71, 287–291. [Google Scholar] [CrossRef]
- Lutz, D.L.; Freitas, S.C. Valor Nutricional. In Pimentas Capsicum; Ribeiro, C.S.C., Lopes, C.A., Carvalho, S.I.C., Henz, G.P., Reifschneider, F.J.B., Eds.; Empresa Brasileira de Pesquisas Agropecuárias: Brasília, Brasil, 2008; pp. 31–38. [Google Scholar]
- Pino, J.; González, M.; Ceballos, L.; Centurión-Yah, A.R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- de Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative study of capsaicinoid composition in Capsicum peppers grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef]
- Lopes, C.A. Ardume, Picância, Pungência. In Pimentas Capsicum; Ribeiro, C.S.C., Lopes, C.A., Carvalho, S.I.C., Henz, G.P., Reifschneider, F.J.B., Eds.; Empresa Brasileira de Pesquisas Agropecuárias: Brasília, Brasil, 2008; pp. 31–38. [Google Scholar]
- Teodoro, A.F.P.; Alves, R.D.B.; Ribeiro, L.B.; Reis, K.; Reifschneider, F.J.B.; Fonseca, M.E.D.N.; Silva, J.P.D.; Agostini-Costa, T.D.S. Vitamin C content in Habanero pepper accessions (Capsicum chinense). Hortic. Bras. 2013, 31, 59–62. [Google Scholar] [CrossRef]
- Topuz, A.; Dincer, C.; Özdemir, K.S.; Feng, H.; Kushad, M. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv., Jalapeno). Food Chem. 2011, 129, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; De Luca, D.; Menichini, F. Air-dried Capsicum annuum var. acuminatum medium and big: Determination of bioactive constituents, antioxidant activity and carbohydrate-hydrolyzing enzymes inhibition. Food Res. Int. 2012, 45, 170–176. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process. 2011, 89, 504–513. [Google Scholar] [CrossRef]
- Olguín-Rojas, J.A.; Vázquez-León, L.A.; Salgado-Cervantes, M.A.; Barbero, G.F.; Díaz-Pacheco, A.; García-Alvarado, M.A.; Rodríguez Jimenes, G.C. Water and phytochemicals dynamic during drying of red habanero chili pepper (Capsicum chinense) slices. Rev. Mex. Ing. Quim 2019. accepted. [Google Scholar]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Pino, J.; Sauri-Duch, E.; Marbot, R. Changes in volatile compounds of Habanero chile pepper (Capsicum chinense Jack. cv. Habanero) at two ripening stages. Food Chem. 2006, 94, 394–398. [Google Scholar] [CrossRef]
- Lannes, S.D.; Finger, F.L.; Schuelter, A.R.; Casali, V.W.D. Growth and quality of Brazilian accessions of Capsicum chinense fruits. Sci. Hort. 2007, 112, 266–270. [Google Scholar] [CrossRef]
- Estrada, B.; Pomar, F.; Díaz, J.; Merino, F.; Bernal, M.A. Effects of mineral fertilizer supplementation on fruit development and pungency in ‘Padrón’ peppers. J. Hortic. Sci. Biotechnol. 1998, 73, 493–497. [Google Scholar] [CrossRef]
- Kehie, M.; Kumaria, S.; Tandon, P. Osmotic stress induced-capsaicin production in suspension cultures of Capsicum chinense Jacq.cv. Naga King Chili. Acta Physiol. Plant. 2012, 34, 2039–2044. [Google Scholar] [CrossRef]
- Ruiz-Lau, N.; Medina-Lara, F.; Minero-García, Y.; Zamudio-Moreno, E.; Guzmán-Antonio, A.; Echevarría-Machado, I.; Martínez-Estévez, M. Water Deficit Affects the Accumulation of Capsaicinoids in Fruits of Capsicum chinense Jacq. HortScience 2011, 46, 487–492. [Google Scholar] [CrossRef]
- Antonious, G.F.; Berke, T.; Jarret, R.L. Pungency in Capsicum chinense: Variation among countries of origin. J. Environ. Sci. Health Part B 2009, 44, 179–184. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in Capsaicinoids during Development, Maturation, and Senescence of Chile Peppers and Relation with Peroxidase Activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Barbero, G.F.; de Aguiar, A.C.; Carrera, C.; Olachea, Á.; Ferreiro-González, M.; Martínez, J.; Palma, M.; Barroso, C.G. Evolution of Capsaicinoids in Peter Pepper (Capsicum annuum var. annuum) during Fruit Ripening. Chem. Biodivers. 2016, 13, 1068–1075. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.; Patil, B.S. Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. J. Food Compos. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderon, A.A.; Ferrer, M.A.; Merino de Caceres, F.; Ros Barcelo, A. Oxidation of Capsaicin and Capsaicin Phenolic Precursors by the Basic Peroxidase Isoenzyme B6 from Hot Pepper. J. Agric. Food Chem. 1995, 43, 352–355. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderon, A.A.; Pedreno, M.A.; Munoz, R.; Ros Barcelo, A.; Merino de Caceres, F. Capsaicin oxidation by peroxidase from Capsicum annuum (variety annuum) fruits. J. Agric. Food Chem. 1993, 41, 1041–1044. [Google Scholar] [CrossRef]
- Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef]
- Munting, A.J. Development of flower and fruit of Capsicum annuum L. Acta Bot. Neerl. 1974, 23, 415–432. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, J.A.; Hernández, M.S.; Melgarejo, L.M.; Martínez, O.; Fernández-Trujillo, J.P. Physiological behavior and quality traits during fruit growth and ripening of four Amazonic hot pepper accessions. J. Sci. Food Agric. 2008, 88, 847–857. [Google Scholar] [CrossRef]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, A.M.; Powers, J.J. Sensory Properties of Naturally Occurring Capsaicinoids. J. Food Sci. 1988, 53, 902–905. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Application of Hansch’s Model to capsaicinoids and capsinoids: A study using the quantitative structure−activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Zewdie, Y.; Bosland, P.W. Evaluation of genotype, environment, and genotype-by-environment interaction for capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]

| Dpa * | ‘Bode’ | ‘Habanero’ | ‘Habanero Roxo’ |
|---|---|---|---|
| 13 | Green | Green | Green |
| 20 | Green | Green | Green |
| 27 | Green | Green | Green |
| 34 | Green | Green | Green |
| 41 | Green/yellow | Green/yellow | Green/violet |
| 48 | Orange | Orange | Violet |
| 55 | Orange | Orange | Violet |
| 62 | Orange | Orange | Violet |
| 69 | Orange | Orange | Violet |
| 76 | Orange | Over-ripening | Over-ripening |
| 83 | Over-ripening |
| ‘Bode’ | ‘Habanero’ | ‘Habanero Roxo’ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dpa | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC |
| 13 | 0.001 ± 0.000 | 0.058 ± 0.001 | 0.024 ± 0.002 | 0.002 ± 0.001 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.125 ± 0.002 | 0.026 ± 0.002 | 0.002 ± 0.000 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.056 ± 0.021 | 0.014 ± 0.002 | 0.002 ± 0.001 | 0.000 ± 0.000 |
| 20 | 0.001 ± 0.000 | 0.190 ± 0.003 | 0.039 ± 0.003 | 0.003 ± 0.001 | 0.001 ± 0.000 | 0.002 ± 0.000 | 0.291 ± 0.024 | 0.057 ± 0.007 | 0.005 ± 0.000 | 0.001 ± 0.000 | 0.003 ± 0.000 | 0.324 ± 0.013 | 0.073 ± 0.007 | 0.005 ± 0.001 | 0.002 ± 0.000 |
| 27 | 0.001 ± 0.000 | 0.325 ± 0.004 | 0.060 ± 0.003 | 0.006 ± 0.001 | 0.001 ± 0.000 | 0.009 ± 0.000 | 0.646 ± 0.038 | 0.120 ± 0.011 | 0.009 ± 0.001 | 0.001 ± 0.000 | 0.003 ± 0.000 | 0.769 ± 0.043 | 0.152 ± 0.023 | 0.008 ± 0.002 | 0.005 ± 0.000 |
| 34 | 0.001 ± 0.000 | 0.496 ± 0.002 | 0.087 ± 0.003 | 0.007 ± 0.001 | 0.002 ± 0.000 | 0.015 ± 0.001 | 1.063 ± 0.079 | 0.193 ± 0.004 | 0.012 ± 0.001 | 0.001 ± 0.000 | 0.009 ± 0.001 | 1.123 ± 0.035 | 0.168 ± 0.022 | 0.006 ± 0.001 | 0.004 ± 0.000 |
| 41 | 0.002 ± 0.000 | 0.480 ± 0.006 | 0.077 ± 0.007 | 0.006 ± 0.001 | 0.001 ± 0.000 | 0.017 ± 0.001 | 0.957 ± 0.049 | 0.192 ± 0.013 | 0.011 ± 0.001 | 0.002 ± 0.000 | 0.013 ± 0.001 | 1.926 ± 0.065 | 0.249 ± 0.016 | 0.009 ± 0.003 | 0.005 ± 0.000 |
| 48 | 0.003 ± 0.000 | 0.434 ± 0.024 | 0.085 ± 0.006 | 0.004 ± 0.001 | 0.001 ± 0.000 | 0.018 ± 0.002 | 0.932 ± 0.040 | 0.182 ± 0.013 | 0.009 ± 0.000 | 0.002 ± 0.000 | 0.014 ± 0.002 | 1.997 ± 0.046 | 0.237 ± 0.019 | 0.015 ± 0.001 | 0.003 ± 0.000 |
| 55 | 0.003 ± 0.000 | 0.510 ± 0.010 | 0.087 ± 0.007 | 0.005 ± 0.001 | 0.001 ± 0.000 | 0.020 ± 0.002 | 1.110 ± 0.075 | 0.187 ± 0.009 | 0.007 ± 0.000 | 0.002 ± 0.000 | 0.014 ± 0.002 | 2.126 ± 0.021 | 0.249 ± 0.040 | 0.015 ± 0.003 | 0.004 ± 0.000 |
| 62 | 0.003 ± 0.000 | 0.466 ± 0.014 | 0.094 ± 0.009 | 0.003 ± 0.001 | 0.001 ± 0.000 | 0.019 ± 0.002 | 1.102 ± 0.025 | 0.183 ± 0.008 | 0.009 ± 0.000 | 0.002 ± 0.000 | 0.028 ± 0.002 | 2.630 ± 0.043 | 0.350 ± 0.032 | 0.021 ± 0.001 | 0.006 ± 0.000 |
| 69 | 0.005 ± 0.000 | 0.596 ± 0.008 | 0.111 ± 0.008 | 0.006 ± 0.001 | 0.001 ± 0.000 | 0.016 ± 0.002 | 1.038 ± 0.079 | 0.165 ± 0.013 | 0.005 ± 0.001 | 0.002 ± 0.000 | 0.027 ± 0.002 | 2.594 ± 0.054 | 0.376 ± 0.014 | 0.020 ± 0.003 | 0.004 ± 0.000 |
| 76 | 0.005 ± 0.000 | 0.590 ± 0.014 | 0.105 ± 0.011 | 0.004 ± 0.001 | 0.001 ± 0.000 | 0.013 ± 0.002 | 1.051 ± 0.057 | 0.159 ± 0.008 | 0.004 ± 0.000 | 0.001 ± 0.000 | 0.036 ± 0.002 | 3.282 ± 0.077 | 0.511 ± 0.030 | 0.023 ± 0.004 | 0.006 ± 0.001 |
| 83 | 0.006 ± 0.000 | 0.832 ± 0.008 | 0.150 ± 0.008 | 0.009 ± 0.001 | 0.001 ± 0.000 | ||||||||||
| ‘Bode’ | ‘Habanero’ | ‘Habanero Roxo’ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dpa | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC |
| 13 | 0.60 ± 0.20 | 67.84 ± 1.24 | 27.67 ± 0.95 | 2.70 ± 0.34 | 1.20 ± 0.06 | 0.58 ± 0.03 | 80.64 ± 1.02 | 17.12 ± 1.04 | 1.45 ± 0.01 | 0.21 ± 0.01 | 0.87 ± 0.03 | 80.78 ± 1.01 | 15.93 ± 1.04 | 2.24 ± 0.06 | 0.18 ± 0.06 |
| 20 | 0.35 ± 0.07 | 81.10 ± 1.25 | 16.64 ± 0.76 | 1.39 ± 0.38 | 0.52 ± 0.03 | 0.58 ± 0.01 | 81.86 ± 0.51 | 15.99 ± 0.44 | 1.42 ± 0.02 | 0.15 ± 0.01 | 0.65 ± 0.01 | 79.70 ± 2.94 | 17.84 ± 2.81 | 1.32 ± 0.14 | 0.49 ± 0.01 |
| 27 | 0.30 ± 0.04 | 82.65 ± 1.00 | 15.24 ± 0.44 | 1.46 ± 0.22 | 0.36 ± 0.02 | 1.12 ± 0.01 | 82.35 ± 0.02 | 15.27 ± 0.01 | 1.12 ± 0.03 | 0.14 ± 0.01 | 0.37 ± 0.01 | 82.11 ± 1.40 | 16.13 ± 1.28 | 0.82 ± 0.01 | 0.57 ± 0.03 |
| 34 | 0.24 ± 0.03 | 83.71 ± 1.64 | 14.58 ± 0.44 | 1.22 ± 0.15 | 0.26 ± 0.01 | 1.18 ± 0.02 | 82.79 ± 0.29 | 15.01 ± 0.23 | 0.91 ± 0.02 | 0.11 ± 0.01 | 0.64 ± 0.01 | 85.80 ± 1.73 | 12.76 ± 1.03 | 0.48 ± 0.01 | 0.32 ± 0.01 |
| 41 | 0.30 ± 0.03 | 84.73 ± 1.01 | 13.63 ± 0.93 | 1.11 ± 0.16 | 0.23 ± 0.01 | 1.45 ± 0.01 | 81.22 ± 0.44 | 16.29 ± 0.41 | 0.90 ± 0.01 | 0.15 ± 0.01 | 0.58 ± 0.02 | 87.53 ± 0.55 | 11.29 ± 0.28 | 0.39 ± 0.07 | 0.21 ± 0.01 |
| 48 | 0.59 ± 0.01 | 82.48 ± 1.31 | 16.10 ± 0.74 | 0.67 ± 0.15 | 0.16 ± 0.01 | 1.60 ± 0.01 | 81.50 ± 0.40 | 15.92 ± 0.40 | 0.81 ± 0.00 | 0.17 ± 0.01 | 0.62 ± 0.01 | 88.15 ± 0.66 | 10.46 ± 0.52 | 0.66 ± 0.06 | 0.12 ± 0.01 |
| 55 | 0.45 ± 0.02 | 84.25 ± 1.00 | 14.28 ± 0.34 | 0.88 ± 0.17 | 0.14 ± 0.01 | 1.50 ± 0.02 | 83.74 ± 1.02 | 14.13 ± 0.05 | 0.50 ± 0.00 | 0.13 ± 0.01 | 0.58 ± 0.02 | 88.32 ± 1.66 | 10.30 ± 1.37 | 0.63 ± 0.05 | 0.16 ± 0.01 |
| 62 | 0.47 ± 0.02 | 82.16 ± 1.84 | 16.62 ± 1.10 | 0.57 ± 0.12 | 0.18 ± 0.01 | 1.47 ± 0.01 | 83.79 ± 1.00 | 13.90 ± 0.32 | 0.68 ± 0.01 | 0.16 ± 0.01 | 0.92 ± 0.01 | 86.67 ± 1.02 | 11.51 ± 0.73 | 0.69 ± 0.01 | 0.21 ± 0.01 |
| 69 | 0.66 ± 0.02 | 83.04 ± 1.71 | 15.37 ± 0.64 | 0.78 ± 0.12 | 0.15 ± 0.01 | 1.33 ± 0.01 | 84.62 ± 1.20 | 13.44 ± 0.27 | 0.43 ± 0.01 | 0.19 ± 0.01 | 0.89 ± 0.01 | 85.86 ± 0.43 | 12.45 ± 0.14 | 0.66 ± 0.02 | 0.14 ± 0.01 |
| 76 | 0.67 ± 0.02 | 83.64 ± 1.89 | 14.94 ± 0.80 | 0.63 ± 0.08 | 0.12 ± 0.01 | 1.04 ± 0.02 | 85.56 ± 0.32 | 12.95 ± 0.21 | 0.35 ± 0.01 | 0.11 ± 0.01 | 0.93 ± 0.02 | 85.09 ± 0.62 | 13.23 ± 0.37 | 0.60 ± 0.03 | 0.15 ± 0.01 |
| 83 | 0.59 ± 0.09 | 83.37 ± 3.17 | 15.04 ± 1.34 | 0.85 ± 0.04 | 0.14 ± 0.01 | ||||||||||
| ‘Bode’ | ‘Habanero’ | ‘Habanero Roxo’ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dpa | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC | n-DHC | C | DHC | h-C | h-DHC |
| 13 | 8.7 ± 1.1 | 7.0 ± 0.1 | 15.8 ± 0.2 | 26.8 ± 0.6 | 66.3 ± 1.2 | 4.5 ± 0.1 | 11.1 ± 0.4 | 13.6 ± 0.3 | 19.3 ± 0.3 | 13.4 ± 0.5 | 1.6 ± 0.1 | 1.7 ± 0.6 | 2.6 ± 2.2 | 7.2 ± 3.7 | 2.2 ± 1.5 |
| 20 | 13.8 ± 2.9 | 22.8 ± 0.1 | 25.9 ± 0.1 | 37.8 ± 3.3 | 79.4 ± 1.3 | 10.4 ± 0.2 | 25.9 ± 1.0 | 29.2 ± 0.4 | 43.2 ± 0.1 | 21.9 ± 0.8 | 7.6 ± 1.4 | 9.9 ± 0.2 | 14.3 ± 2.5 | 23.3 ± 2.3 | 30.7 ± 0.6 |
| 27 | 19.6 ± 2.7 | 39.0 ± 0.1 | 39.9 ± 0.9 | 67.0 ± 3.9 | 91.9 ± 0.5 | 44.1 ± 1. | 57.5 ± 0.9 | 61.6 ± 1.4 | 75.1 ± 2.6 | 44.9 ± 0.9 | 9.8 ± 2.0 | 23.4 ± 0.8 | 29.6 ± 2.8 | 33.3 ± 5.8 | 82.0 ± 1.0 |
| 34 | 23.6 ± 2.6 | 59.7 ± 0.3 | 57.6 ± 0.4 | 84.5 ± 1.8 | 100.0 ± 0.0 | 76.3 ± 2.0 | 94.5 ± 2.7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 57.4 ± 1.7 | 21.9 ± 2.1 | 34.2 ± 0.3 | 32.7 ± 2.4 | 27.1 ± 5.0 | 64.9 ± 1.1 |
| 41 | 28.3 ± 2.4 | 57.7 ± 0.2 | 51.5 ± 2.1 | 73.6 ± 3.1 | 83.7 ± 1.1 | 86.0 ± 3.7 | 85.1 ± 1.0 | 98.6 ± 2.1 | 90.6 ± 1.1 | 73.1 ± 2.7 | 35.2 ± 2.4 | 58.7 ± 0.6 | 48.7 ± 0.3 | 37.0 ± 9.8 | 70.1 ± 0.6 |
| 48 | 52.4 ± 1.6 | 52.1 ± 2.4 | 56.4 ± 2.5 | 41.3 ± 4.5 | 53.6 ± 3.0 | 92.4 ± 2.2 | 82.9 ± 1.0 | 93.6 ± 3.2 | 79.0 ± 0.4 | 79.9 ± 2.8 | 39.4 ± 1.6 | 60.8 ± 0.0 | 46.4 ± 1.0 | 64.6 ± 1.3 | 40.9 ± 1.8 |
| 55 | 46.2 ± 1.8 | 61.3 ± 0.6 | 57.6 ± 1.1 | 62.1 ± 2.4 | 55.5 ± 2.9 | 100.0 ± 0.0 | 100.0 ± 1.0 | 96.2 ± 3.1 | 56.8 ± 2.1 | 75.1 ± 2.6 | 38.5 ± 4.4 | 64.8 ± 0.9 | 48.5 ± 5.0 | 65.9 ± 3.7 | 60.1 ± 0.3 |
| 62 | 44.6 ± 1.9 | 56.0 ± 1.1 | 62.9 ± 1.1 | 37.5 ± 4.0 | 67.2 ± 2.1 | 97.6 ± 0.3 | 98.1 ± 2.3 | 94.0 ± 1.3 | 76.1 ± 0.1 | 88.6 ± 0.3 | 78.1 ± 3.0 | 80.1 ± 0.6 | 68.4 ± 2.3 | 90.5 ± 5.9 | 100.0 ± 0.0 |
| 69 | 79.5 ± 0.7 | 71.7 ± 0.3 | 73.5 ± 3.2 | 65.7 ± 5.7 | 70.1 ± 1.9 | 81.8 ± 3.7 | 92.2 ± 2.8 | 84.6 ± 1.3 | 44.9 ± 1.2 | 100.0 ± 0.0 | 75.0 ± 2.2 | 79.1 ± 0.2 | 73.8 ± 1.6 | 85.5 ± 4.6 | 65.8 ± 0.5 |
| 76 | 79.5 ± 0.7 | 70.9 ± 1.0 | 70.2 ± 1.1 | 52.1 ± 2.3 | 53.8 ± 3.0 | 64.1 ± 1.3 | 93.5 ± 1.2 | 81.7 ± 2.3 | 36.5 ± 1.7 | 57.6 ± 3.9 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 89.5 ± 5.0 |
| 83 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 93.0 ± 0.5 | ||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodríguez-Jimenes, G.d.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030141
Olguín-Rojas JA, Fayos O, Vázquez-León LA, Ferreiro-González M, Rodríguez-Jimenes GdC, Palma M, Garcés-Claver A, Barbero GF. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy. 2019; 9(3):141. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030141
Chicago/Turabian StyleOlguín-Rojas, José Arturo, Oreto Fayos, Lucio Abel Vázquez-León, Marta Ferreiro-González, Guadalupe del Carmen Rodríguez-Jimenes, Miguel Palma, Ana Garcés-Claver, and Gerardo F. Barbero. 2019. "Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq." Agronomy 9, no. 3: 141. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030141