A Method for a Fast Evaluation of the Biostimulant Potential of Different Natural Extracts for Promoting Growth or Tolerance against Abiotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candidate Biostimulants
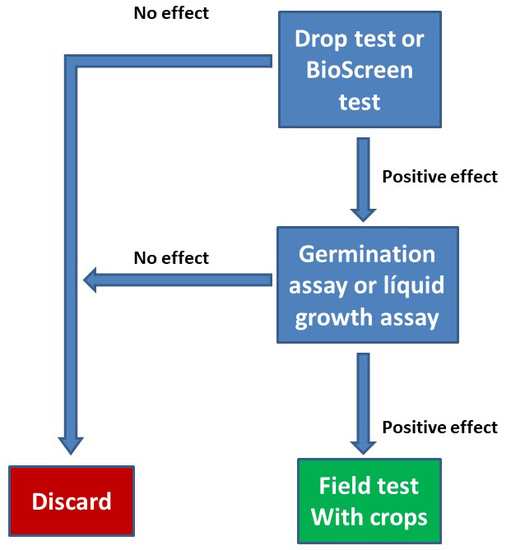
2.2. Experimental Setup
2.3. Drop Test Yeast Assays
2.4. BioScreen® Assays
2.5. Plant Assays
3. Results
3.1. Determination of the Toxic Dose of the Products
3.2. Determination of the Biostimulant Effect in Yeast
3.3. Evaluation of the Biostimulant Effect of the Different Product in Yeast Under Continuous Growth
3.4. Biostimulant Effect of the Tested Products in Arabidopsis Germination and Early Development
3.5. Biostimulant Effect of the Tested Products in Mass Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christou, P. Sustainable Food Production; Springer: New York, NY, USA, 2013. [Google Scholar]
- Park, M. The Fertilizer Industry; International Fertilizer Industry Association: Cambridge, MA, USA, 2001. [Google Scholar]
- Filatov, V.P.; Ibiber, V.K. Voprosu o prirode biogennykh stymulyatorov perezhivayushchikh rastitelnykh tkanei. Doklady Akademii Nauk Sssr. 1948, 62, 259–262. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput Plant Phenotyping for Developing Novel Biostimulants: From Lab to Field or From Field to Lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. (Eds.) Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Food Products Press: New York, NY, USA, 2005; p. 725. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Taibi, K.; del Campo, A.; Vilagrosa, A.; Belles, J.M.; Lopez-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought tolerance in Pinus halepensis seed sources as identified by distinctive physiological and molecular markers. Front. Plant Sci. 2017, 8, 1202. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mulet, J.M.; Alemany, B.; Ros, R.; Calvete, J.J.; Serrano, R. Expression of a plant serine O-acetyltransferase in Saccharomyces cerevisiae confers osmotic tolerance and creates an alternative pathway for cysteine biosynthesis. Yeast 2004, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Arisi, A.C.M.; Jouanin, L.; Valadier, M.H.; Roux, Y.; Foyer, C.H. The role of glycine in determining the rate of glutathione synthesis in poplar. Possible implications for glutathione production during stress. Physiol. Plant. 1997, 100, 255–263. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Traon, D.; Amat, L.; Zotz, F.; du Jardin, P. A Legal Framework for Plant Biostimulants and Agronomic Fertiliser Additives in the EU; European Comission: Brussels, Belgium, 2014. [Google Scholar]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; Fink, G. Yeast: An Experimental Organism for 21st Century Biology. Genetics 2011, 189, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsburg, S. The art and design of genetic screens: Yeast. Nat. Rev. Genet. 2001, 2, 659–668. [Google Scholar] [CrossRef]
- Montesinos, C.; Gaxiola, R.; Ríos, G.; Forment, J.; Leube, M.; Mulet, J.M.; Naranjo, M.A.; Roldán, M.; Vicente, O.; Kanhonou, R.A.; et al. Functional genomics of salt tolerance: The yeast overexpression approach. In Proceedings of the International Symposium on Managing Greenhouse Crops in Saline Environment, Pisa, Italy, 9–12 July 2003; pp. 31–38. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Provart, N.J.; Alonso, J.; Assmann, S.M.; Bergmann, D.; Brady, S.M.; Brkljacic, J.; Browse, J.; Chapple, C.; Colot, V.; Cutler, S.; et al. 50 years of Arabidopsis research: Highlights and future directions. New Phytol. 2016, 209, 921–944. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.; Cabedo, M.; Rull, B.; Yenush, L.; Serrano, R.; Mulet, J.M. Role of the yeast multidrug transporter Qdr2 in cation homeostasis and the oxidative stress response. FEMS Yeast Res. 2013, 13, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Bissoli, G.; Niñoles, R.; Fresquet, S.; Palombieri, S.; Bueso, E.; Rubio, L.; García-Sánchez, M.J.; Fernández, J.A.; Mulet, J.M.; Serrano, R. Peptidyl-prolyl cis-trans isomerase ROF2 modulates intracellular pH homeostasis in Arabidopsis. Plant J. 2012, 70, 704–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicent, I.; Navarro, A.; Mulet, J.M.; Sharma, S.; Serrano, R. Uptake of inorganic phosphate is a limiting factor for Saccharomyces cerevisiae during growth at low temperatures. FEMS Yeast Res. 2015, 15, fov008. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Saa, S.; Olivos-Del Rio, A.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef]






| Product Number | Description | Major Component |
|---|---|---|
| 1 | Extract from fragaria X ananassa plants | Polysachharides |
| 2 | Extract from Ascophyllum nodosum | Polysachharides |
| 3 | Concentrated extract from Ascophyllum nodosum | Polysachharides |
| 4 | Extract from chitosan | Polysachharides |
| 5 | Extract from Ascophyllum nodosum and added amino acids | Polysaccharides |
| 6 | Whey | Proteins |
| 7 | Extract from Arthrospira platensis and Arthrospira maxima | Polysaccharides |
| 8 | Humic and fulvic acid | Heterogeneus organic molecules |
| 9 | Extract from Equisetum arvense | Polysaccharides |
| 10 | Extract from compost | Heterogeneus organic molecules |
| 11 | Extract from Ecklonia maxima | Polysaccharides |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saporta, R.; Bou, C.; Frías, V.; Mulet, J.M. A Method for a Fast Evaluation of the Biostimulant Potential of Different Natural Extracts for Promoting Growth or Tolerance against Abiotic Stress. Agronomy 2019, 9, 143. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030143
Saporta R, Bou C, Frías V, Mulet JM. A Method for a Fast Evaluation of the Biostimulant Potential of Different Natural Extracts for Promoting Growth or Tolerance against Abiotic Stress. Agronomy. 2019; 9(3):143. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030143
Chicago/Turabian StyleSaporta, Rubén, Claudia Bou, Victor Frías, and José M. Mulet. 2019. "A Method for a Fast Evaluation of the Biostimulant Potential of Different Natural Extracts for Promoting Growth or Tolerance against Abiotic Stress" Agronomy 9, no. 3: 143. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy9030143