Influence of Mesenchymal Stem Cell Sources on Their Regenerative Capacities on Different Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Proteomic Analysis by iTRAQ Labelling
2.2. Samples
2.3. Cell Isolation and Culture
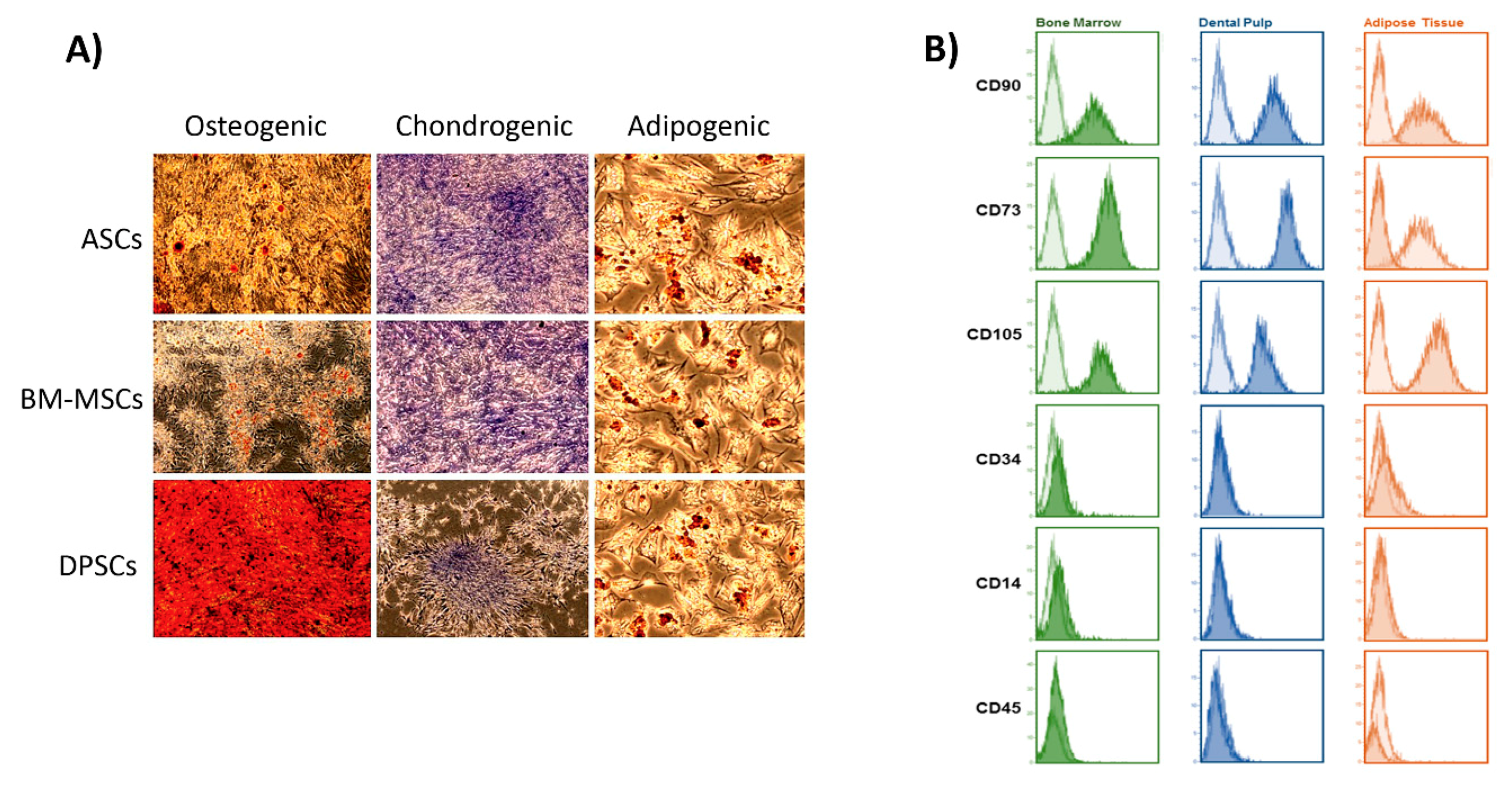
2.4. Cell Characterisation
2.5. Biological Features on the Plastic Surface
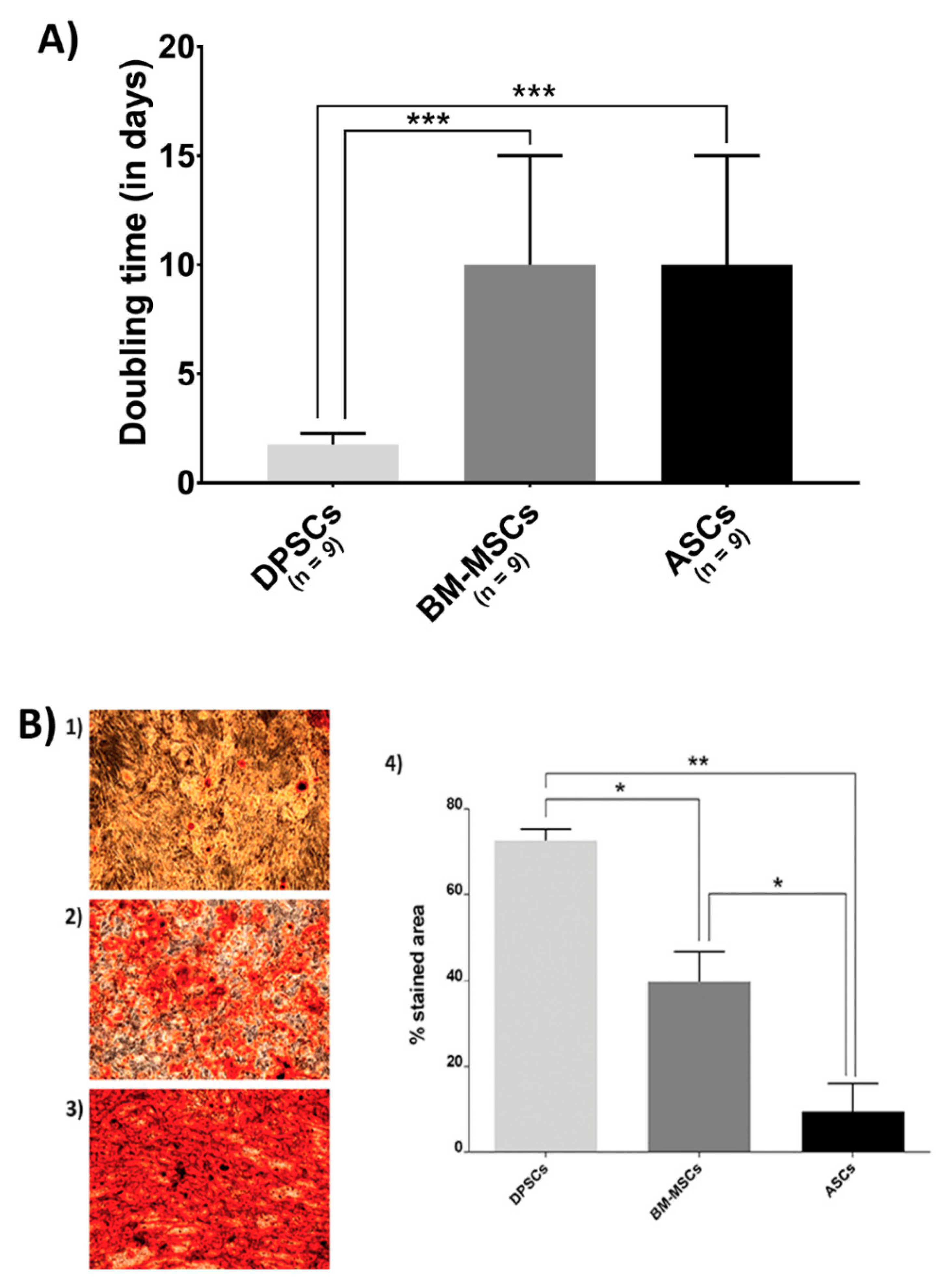
2.5.1. MSC Proliferation Rate
2.5.2. Osteogenic Commitment of Each MSC
2.6. Cell Behaviour on CaPs
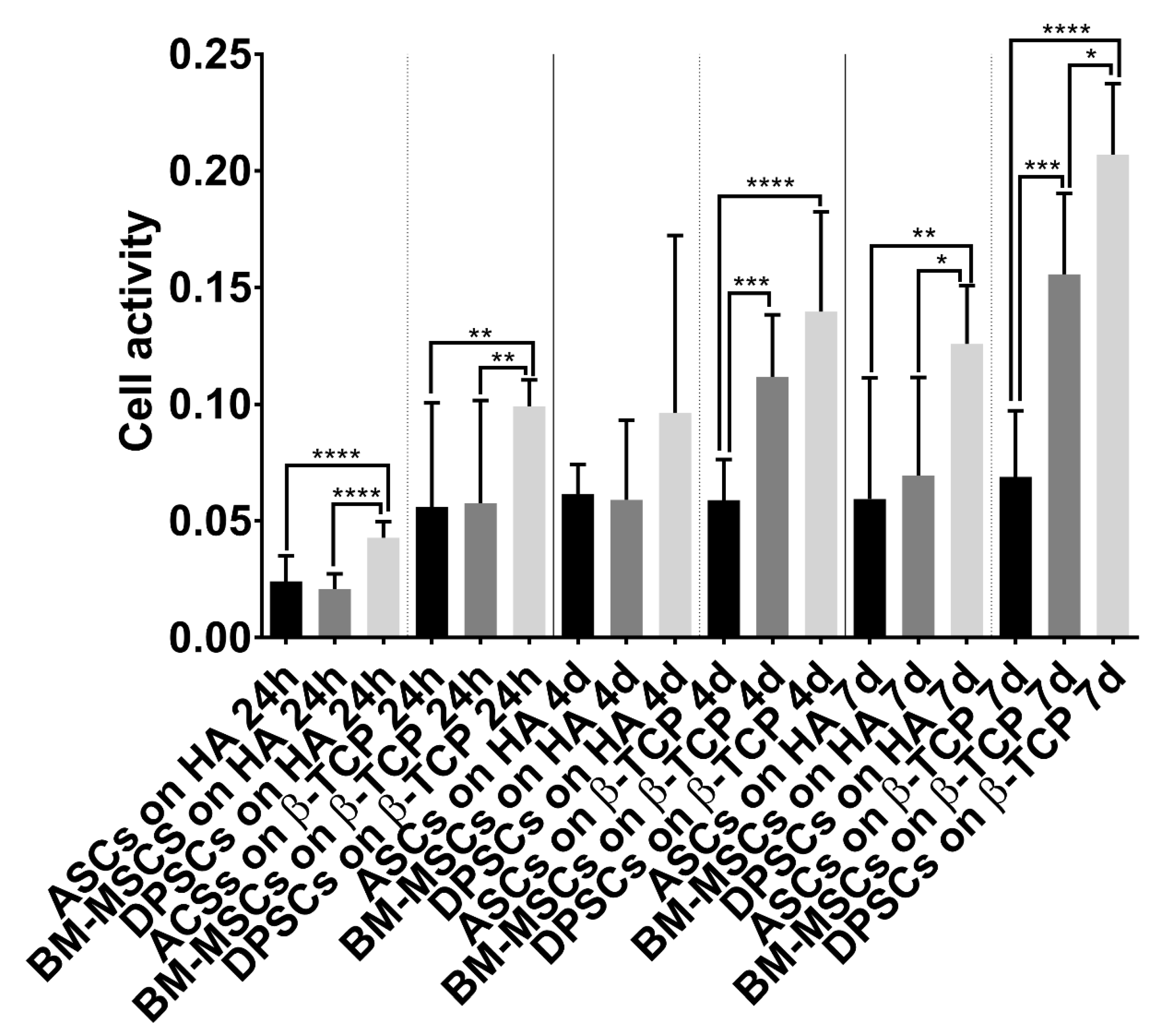
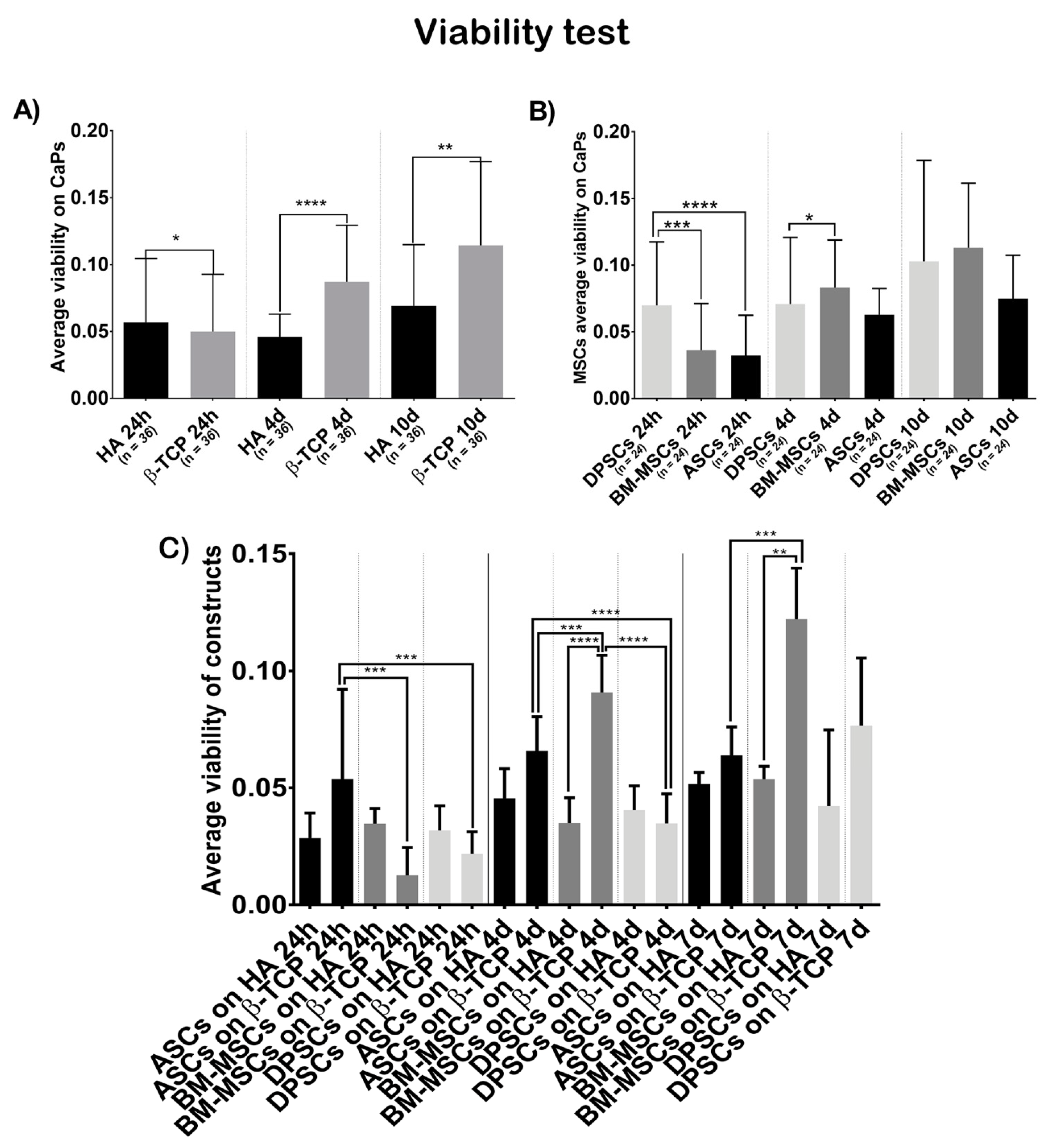
2.6.1. Cell Activity/Viability
2.6.2. Scanning Electronic Microscopy (SEM)
2.6.3. ALP Activity
2.7. Statistical Analysis
3. Results
3.1. iTRAQ Results Analysis
3.2. Biological Features on the Plastic Surface
3.3. Cell Behaviour on CaPs
3.3.1. Viability Test
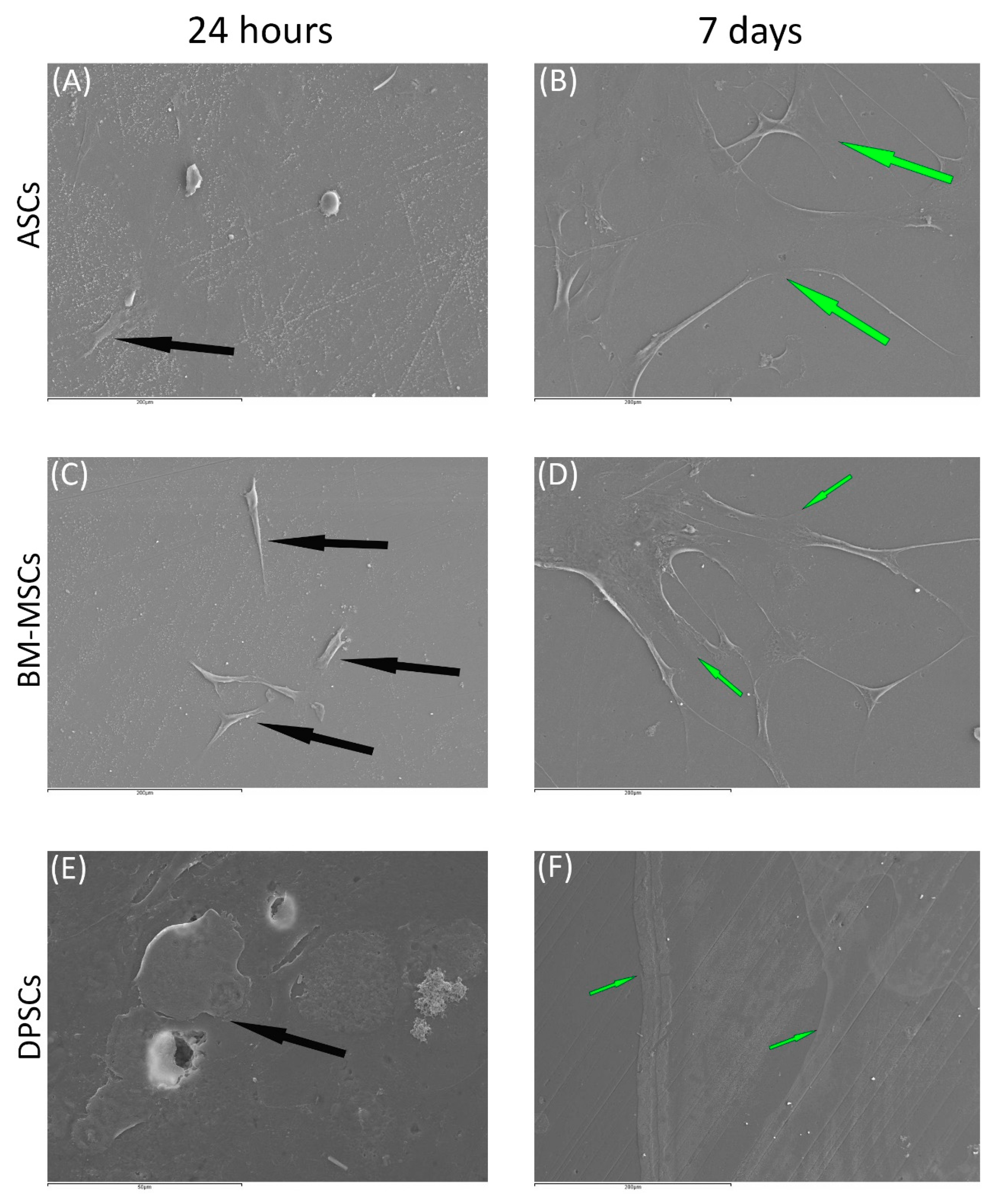
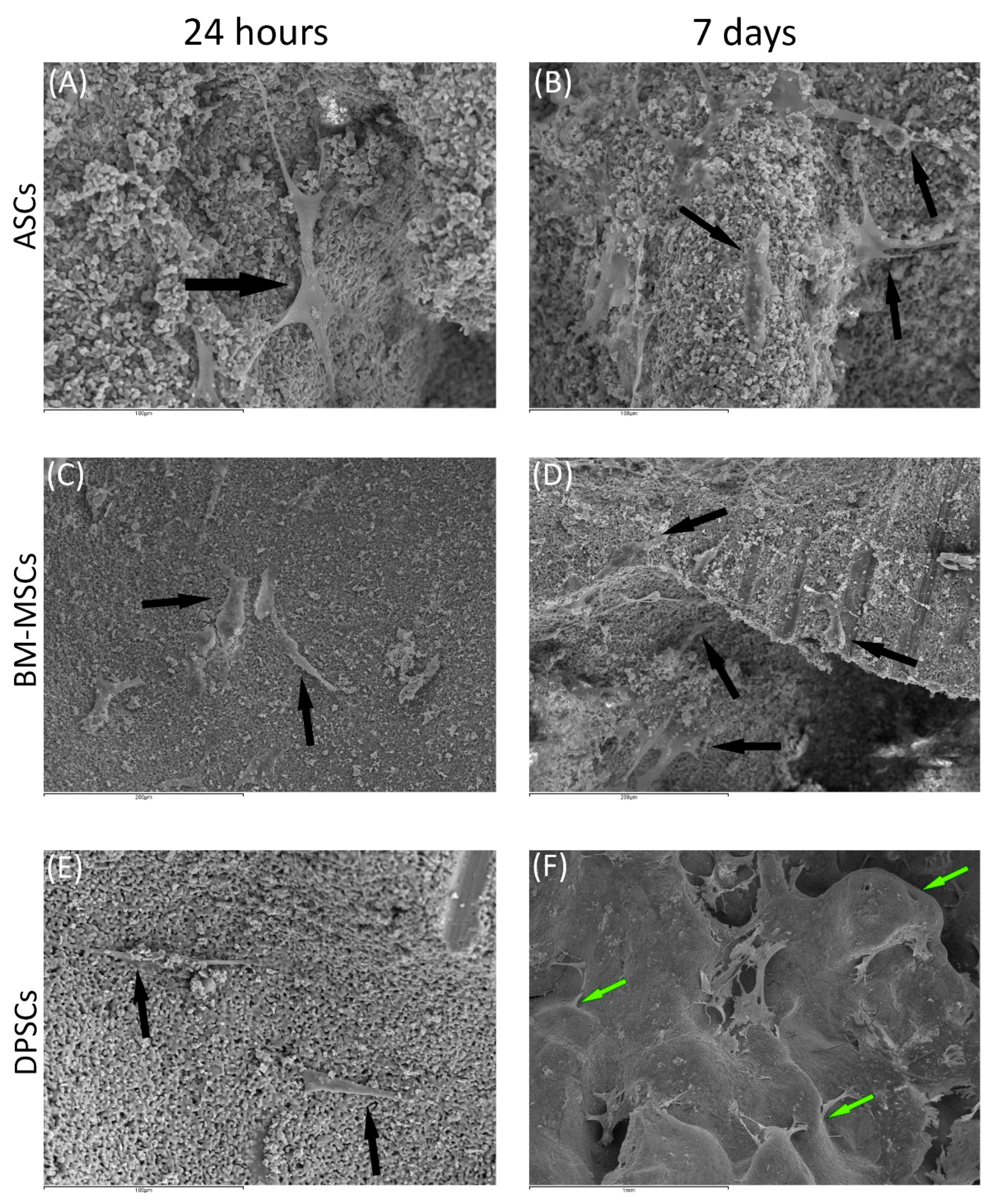
3.3.2. Scanning Electronic Microscopy
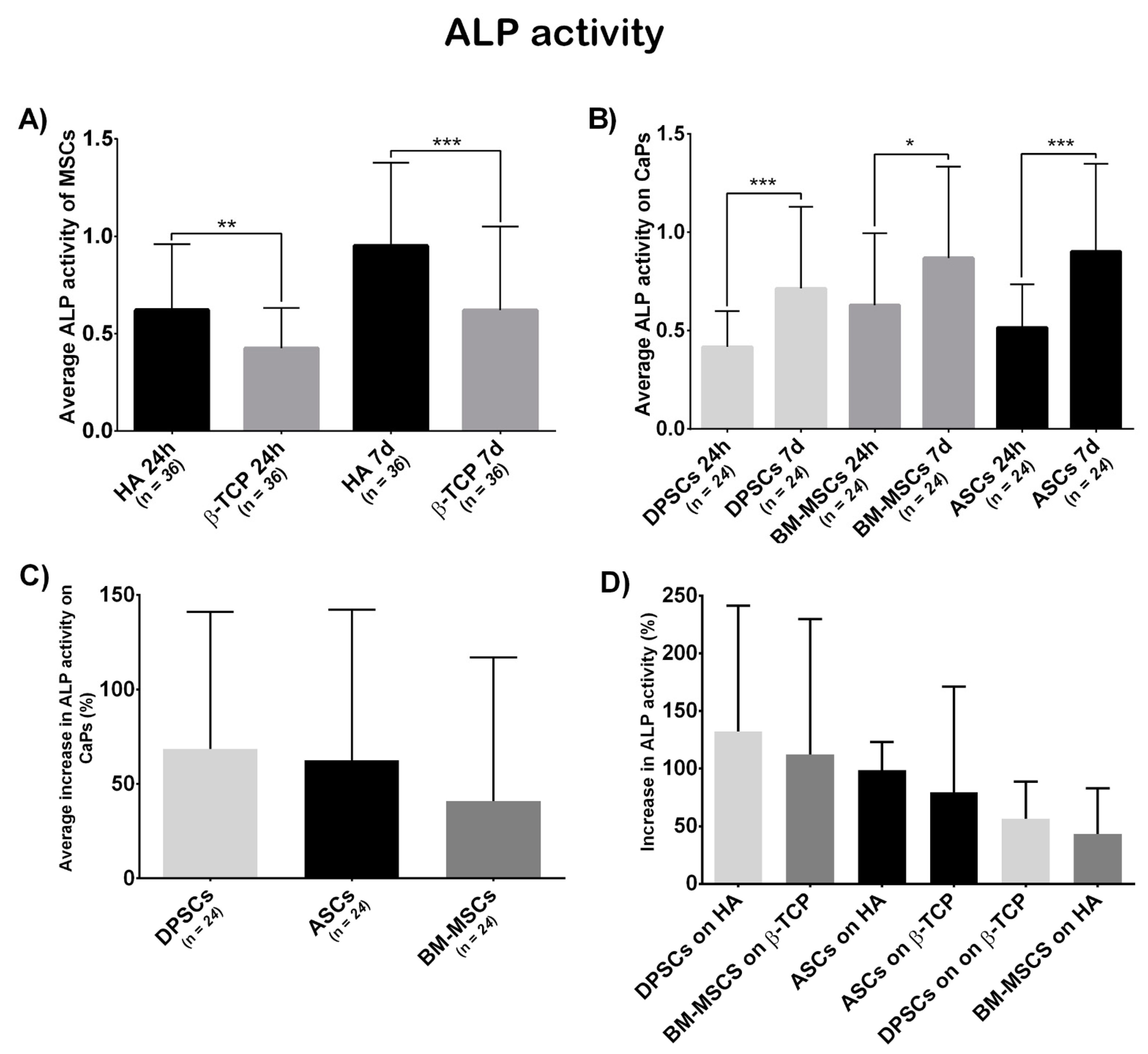
3.3.3. Osteogenic Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in Bone Tissue Regeneration: Stem Cell Therapy, Biofunctionality and Antimicrobial Properties of Novel Materials and Its Evolution. Int. J. Mol. Sci. 2020, 22, 192. [Google Scholar] [CrossRef]
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355S, S7–S21. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Calori, G.M.; Begue, T.; Schmidmaier, G. Bone regeneration strategies: Current trends but what the future holds? Injury 2013, 44, S1–S2. [Google Scholar] [CrossRef]
- Qin, W.; Chen, J.-Y.; Guo, J.; Ma, T.; Weir, M.D.; Guo, D.; Shu, Y.; Lin, Z.-M.; Schneider, A.; Xu, H.H.K. Novel Calcium Phosphate Cement with Metformin-Loaded Chitosan for Odontogenic Differentiation of Human Dental Pulp Cells. Stem Cells Int. 2018, 2018, 7173481. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.W.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Descamps, M.; Boilet, L.; Moreau, G.; Tricoteaux, A.; Lu, J.; Leriche, A.; Lardot, V.; Cambier, F. Processing and properties of biphasic calcium phosphates bioceramics obtained by pressureless sintering and hot isostatic pressing. J. Eur. Ceram. Soc. 2013, 33, 1263–1270. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, K.H.; Jang, I.K.; Lee, M.W.; Kim, H.E.; Yang, M.S.; Eom, Y.; Lee, J.E.; Kim, Y.J.; Yang, S.K.; Jung, H.L.; et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell. Immunol. 2009, 259, 150–156. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, K.; Yue, J.; Meng, S.; Zhang, X. Deconstructing transcriptional variations and their effects on immunomodulatory function among human mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 53. [Google Scholar] [CrossRef]
- Stubbendorff, M.; Deuse, T.; Hua, X.; Phan, T.T.; Bieback, K.; Atkinson, K.; Eiermann, T.H.; Velden, J.; Schröder, C.; Reichenspurner, H.; et al. Immunological Properties of Extraembryonic Human Mesenchymal Stromal Cells Derived from Gestational Tissue. Stem Cells Dev. 2013, 22, 2619–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escacena, N.; Quesada-Hernández, E.; Capilla-Gonzalez, V.; Soria, B.; Hmadcha, A. Bottlenecks in the Efficient Use of Advanced Therapy Medicinal Products Based on Mesenchymal Stromal Cells. Stem Cells Int. 2015, 2015, 895714. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials 2014. [Google Scholar] [CrossRef]
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; De Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell. Mol. Med. 2009, 14, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part B Rev. 2019, 25, 78–88. [Google Scholar] [CrossRef]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Tong, J.; Yang, X.; Zhao, J.; Zheng, Q.; Zhao, G.; Ma, Z. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Lobo, S.E.; Glickman, R.; da Silva, W.N.; Arinzeh, T.L.; Kerkis, I. Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tissue Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef] [Green Version]
- D’Alimonte, I.; Mastrangelo, F.; Giuliani, P.; Pierdomenico, L.; Marchisio, M.; Zuccarini, M.; Di Iorio, P.; Quaresima, R.; Caciagli, F.; Ciccarelli, R. Osteogenic Differentiation of Mesenchymal Stromal Cells: A Comparative Analysis Between Human Subcutaneous Adipose Tissue and Dental Pulp. Stem Cells Dev. 2017, 26, 843–855. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef]
- Hornez, J.C.; Chai, F.; Monchau, F.; Blanchemain, N.; Descamps, M.; Hildebrand, H.F. Biological and physico-chemical assessment of hydroxyapatite (HA) with different porosity. Biomol. Eng. 2007, 24, 505–509. [Google Scholar] [CrossRef]
- Descamps, M.; Duhoo, T.; Monchau, F.; Lu, J.; Hardouin, P.; Hornez, J.C.; Leriche, A. Manufacture of macroporous β-tricalcium phosphate bioceramics. J. Eur. Ceram. Soc. 2008, 28, 149–157. [Google Scholar] [CrossRef]
- Yang, X.-F.; He, X.; He, J.; Zhang, L.-H.; Su, X.-J.; Dong, Z.-Y.; Xu, Y.-J.; Li, Y.; Li, Y.-L. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J. Biomed. Sci. 2011, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.J.; Sonoyama, W.; Chen, J.; Park, S.H. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006, 324, 225–236. [Google Scholar] [CrossRef]
- Gudleviciene, Z.; Kundrotas, G.; Liudkeviciene, R.; Rascon, J.; Jurga, M. Quick and effective method of bone marrow mesenchymal stem cell extraction. Open Med. 2015, 10, 44–49. [Google Scholar] [CrossRef]
- Alkhalil, M.; Smajilagić, A.; Redžić, A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med. Glas. 2015, 12, 27–32. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Tornero-Esteban, P.; Peralta-Sastre, A.; Herranz, E.; Rodríguez-Rodríguez, L.; Mucientes, A.; Abásolo, L.; Marco, F.; Fernández-Gutiérrez, B.; Lamas, J.R. Altered Expression of Wnt Signaling Pathway Components in Osteogenesis of Mesenchymal Stem Cells in Osteoarthritis Patients. PLoS ONE 2015, 10, e0137170. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Wall, M.E.; Rachlin, A.; Otey, C.A.; Loboa, E.G. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am. J. Physiol. Cell Physiol. 2007, 293, C1532–C1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Tay, C.Y.; Leong, W.S.; Tan, S.C.W.; Liao, K.; Tan, L.P. Mechanical behavior of human mesenchymal stem cells during adipogenic and osteogenic differentiation. Biochem. Biophys. Res. Commun. 2010, 393, 150–155. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, L.; Xue, P.; An, Y.; Luo, L.; Zhang, R.; Wu, G.; Wang, Y.; Zhu, H.; Wang, Q. Tumor Necrosis Factor-α Attenuates the Osteogenic Differentiation Capacity of Periodontal Ligament Stem Cells by Activating PERK Signaling. J. Periodontol. 2016, 87, e159–e171. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Romano, C.; Stacchiotti, A.; Pasini, E.; Dioguardi, F.S. Endoplasmic Reticulum Stress and Apoptosis Triggered by Sub-Chronic Lead Exposure in Mice Spleen: A Histopathological Study. Biol. Trace Elem. Res. 2017, 178, 86–97. [Google Scholar] [CrossRef]
- Chen, L.; Shi, K.; Frary, C.E.; Ditzel, N.; Hu, H.; Qiu, W.; Kassem, M. Inhibiting actin depolymerization enhances osteoblast differentiation and bone formation in human stromal stem cells. Stem Cell Res. 2015, 15, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, K.; Akiba, Y.; Akiba, N.; Nagasawa, M.; Cooper, L.F.; Uoshima, K. Insulin-like growth factor binding Protein-3 suppresses osteoblast differentiation via bone morphogenetic protein-2. Biochem. Biophys. Res. Commun. 2018, 507, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.M.; Ahmed, S.; Selvendiran, K.; Kuppusamy, M.L.; Khan, M.; Kuppusamy, P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am. J. Physiol. Physiol. 2010, 299, C1562–C1570. [Google Scholar] [CrossRef] [PubMed]
- Tzur, A.; Kafri, R.; LeBleu, V.S.; Lahav, G.; Kirschner, M.W. Cell Growth and Size Homeostasis in Proliferating Animal Cells. Science 2009, 325, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, Y.; Deng, Z.; Tang, L.; Li, Y.; Shi, J.; Jin, Y. Odontogenic capability: Bone marrow stromal stem cells versus dental pulp stem cells. Biol. Cell 2007, 99, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Wach, T. New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials 2020, 13, 2935. [Google Scholar] [CrossRef]
- Wang, X.; Song, W.; Kawazoe, N.; Chen, G. The osteogenic differentiation of mesenchymal stem cells by controlled cell-cell interaction on micropatterned surfaces. J. Biomed. Mater. Res. Part A 2013, 101, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Wongsupa, N.; Nuntanaranont, T.; Kamolmattayakul, S.; Thuaksuban, N. Biological characteristic effects of human dental pulp stem cells on poly-ε-caprolactone-biphasic calcium phosphate fabricated scaffolds using modified melt stretching and multilayer deposition. J. Mater. Sci. Mater. Med. 2017, 28, 25. [Google Scholar] [CrossRef]
- Dey, K.; Roca, E.; Ramorino, G.; Sartore, L. Progress in the mechanical modulation of cell functions in tissue engineering. Biomater. Sci. 2020, 8, 7033–7081. [Google Scholar] [CrossRef]
- Sabbagh, J.; Ghassibe-Sabbagh, M.; Fayyad-Kazan, M.; Al-Nemer, F.; Fahed, J.C.; Berberi, A.; Badran, B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020, 101, 103413. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Creasey, S.; Sammon, C.; Le Maitre, C.L. Hydroxyapatite nanoparticle injectable hydrogel scaffold to support osteogenic differentiation of human mesenchymal stem cells. Eur. Cell. Mater. 2016, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tsukanaka, M.; Fujibayashi, S.; Otsuki, B.; Takemoto, M.; Matsuda, S. Osteoinductive potential of highly purified porous β-TCP in mice. J. Mater. Sci. Mater. Med. 2015, 26, 132. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.G.; Münchow, E.A.; Tamerler, C.; Bottino, M.C.; Aparicio, C. Harnessing biomolecules for bioinspired dental biomaterials. J. Mater. Chem. B 2020, 8, 8713–8747. [Google Scholar] [CrossRef] [PubMed]

| Donor | Cell Type | Sex | Age |
| Donor 1 | BM-MSC | F | 57 |
| Donor 2 | BM-MSC | M | 56 |
| Donor 3 | BM-MSC | M | 55 |
| Donor 4 | ASC | F | 53 |
| Donor 5 | ASC | F | 56 |
| Donor 6 | ASC | M | 50 |
| Donor 7 | DPSC | F | 44 |
| Donor 8 | DPSC | M | 58 |
| Donor 9 | DPSC | M | 59 |
| Uniprot ID | Gene | Protein | Peptides | Fold Change | p-Value |
|---|---|---|---|---|---|
| AMPN_HUMAN | ANPEP | Aminopeptidase N | 27 | 11.27 | 0.0001 |
| PALLD_HUMAN | PALLD | Palladin | 4 | 4.6989 | 0.0145 |
| K2C1_HUMAN | KRT1 | Keratin, type II cytoskeletal 1 | 11 | 4.6352 | 0.0164 |
| TPM1_HUMAN | TPM1 | Tropomyosin alpha-1 chain | 13 | 4.3652 | 0.0173 |
| TAGL_HUMAN | TAGLN | Transgelin | 13 | 4.2855 | 0.0001 |
| K1C10_HUMAN | KRT10 | Keratin, type I cytoskeletal 10 | 8 | 3.5645 | 0.0085 |
| FRIH_HUMAN | FTH1 | Ferritin heavy chain | 3 | 3.4356 | 0.034 |
| DHB4_HUMAN | HSD17B4 | Peroxisomal multifunctional enzyme type 2 | 5 | 3.4041 | 0.0078 |
| VIME_HUMAN | VIM | Vimentin | 235 | 3.3420 | 0.0291 |
| SQRD_HUMAN | SQRDL | Sulphide:quinone oxidoreductase, mitochondrial | 10 | 2.9923 | 0.0030 |
| PDIA1_HUMAN | P4HB | Protein disulphide-isomerase | 48 | 2.7290 | 0.0028 |
| KPYM_HUMAN | PKM2 | Pyruvate kinase isozymes M1/M2 | 69 | 2.6062 | 0.0123 |
| ATPB_HUMAN | ATP5B | ATP synthase subunit beta, mitochondrial | 21 | 2.5351 | 0.0118 |
| GRP78_HUMAN | HSPA5 | 78 kDa glucose-regulated protein | 53 | 2.5119 | 0.0001 |
| GLCM_HUMAN | GBA | Glucosylceramidase | 4 | 2.4210 | 0.0472 |
| DPYL3_HUMAN | DPYSL3 | Dihydropyrimidinase-related protein 3 | 14 | 2.3768 | 0.0232 |
| SPTB2_HUMAN | SPTBN1 | Spectrin beta chain, brain 1 | 31 | 2.0512 | 0.0251 |
| GDIR1_HUMAN | ARHGDIA | Rho GDP-dissociation inhibitor 1 | 8 | 2.0137 | 0.0105 |
| ACADV_HUMAN | ACADVL | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial | 7 | 1.8707 | 0.0486 |
| ATPA_HUMAN | ATP5A1 | ATP synthase subunit alpha, mitochondrial | 24 | 1.8535 | 0.0070 |
| MYL6_HUMAN | MYL6 | Myosin light polypeptide 6 | 16 | 1.7865 | 0.0487 |
| LMO7_HUMAN | LMO7 | LIM domain only protein 7 | 11 | 1.6144 | 0.0436 |
| 5NTD_HUMAN | NT5E | 5′-nucleotidase | 22 | 1.5704 | 0.0440 |
| FLNA_HUMAN | FLNA | Filamin-A | 126 | 1.5276 | 0.0036 |
| HS90A_HUMAN | HSP90AA1 | Heat shock protein HSP 90-alpha | 29 | 1.5276 | 0.0351 |
| H32_HUMAN | HIST2H3A | Histone H3.2 | 7 | 0.8872 | 0.0298 |
| H2AJ_HUMAN | H2AFJ | Histone H2A.J | 7 | 0.7798 | 0.0365 |
| PDLI7_HUMAN | PDLIM7 | PDZ and LIM domain protein 7 | 1 | 0.7311 | 0.0042 |
| ALDR_HUMAN | AKR1B1 | Aldose reductase | 5 | 0.6918 | 0.0237 |
| FLNC_HUMAN | FLNC | Filamin-C | 14 | 0.6310 | 0.0145 |
| MVP_HUMAN | MVP | Major vault protein | 23 | 0.5649 | 0.0078 |
| VINC_HUMAN | VCL | Vinculin | 23 | 0.5546 | 0.0245 |
| RS15A_HUMAN | RPS15A | 40S ribosomal protein S15a | 4 | 0.5248 | 0.0221 |
| SFPQ_HUMAN | SFPQ | Splicing factor, proline- and glutamine-rich | 3 | 0.4966 | 0.0205 |
| PLIN3_HUMAN | PLIN3 | Perilipin-3 | 14 | 0.4875 | 0.0418 |
| NNMT_HUMAN | NNMT | Nicotinamide N-methyltransferase | 6 | 0.4742 | 0.0400 |
| 1433Z_HUMAN | YWHAZ | 14-3-3 protein zeta/delta | 14 | 0.4699 | 0.0217 |
| PRDX1_HUMAN | PRDX1 | Peroxiredoxin-1 | 21 | 0.4169 | 0.0199 |
| ANX11_HUMAN | ANXA11 | Annexin A11 | 9 | 0.3733 | 0.0014 |
| G6PD_HUMAN | G6PD | Glucose-6-phosphate 1-dehydrogenase | 15 | 0.3664 | 0.0006 |
| HYEP_HUMAN | EPHX1 | Epoxide hydrolase 1 | 7 | 0.3467 | 0.0069 |
| 6PGD_HUMAN | PGD | 6-phosphogluconate dehydrogenase, decarboxylating | 10 | 0.2911 | 0.0040 |
| CALU_HUMAN | CALU | Calumenin | 14 | 0.2655 | 0.0057 |
| NQO1_HUMAN | NQO1 | NAD(P)H dehydrogenase [quinone] 1 | 11 | 0.2512 | 0.0153 |
| TKT_HUMAN | TKT | Transketolase | 21 | 0.1486 | 0.0051 |
| ANXA5_HUMAN | ANXA5 | Annexin A5 | 38 | 0.1294 | 0.0002 |
| ENOA_HUMAN | ENO1 | Alpha-enolase | 54 | 0.1000 | 0.0419 |
| ANXA2_HUMAN | ANXA2 | Annexin A2 | 74 | 0.0871 | 0.0019 |
| IBP3_HUMAN | IGFBP3 | Insulin-like growth factor-binding protein 3 | 5 | 0.0802 | 0.0424 |
| DEST_HUMAN | DSTN | Destrin | 8 | 0.0614 | 0.0245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucientes, A.; Herranz, E.; Moro, E.; González-Corchón, A.; Peña-Soria, M.J.; Abasolo, L.; Rodriguez-Rodriguez, L.; Lamas, J.R.; Fernández-Gutiérrez, B. Influence of Mesenchymal Stem Cell Sources on Their Regenerative Capacities on Different Surfaces. Cells 2021, 10, 481. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10020481
Mucientes A, Herranz E, Moro E, González-Corchón A, Peña-Soria MJ, Abasolo L, Rodriguez-Rodriguez L, Lamas JR, Fernández-Gutiérrez B. Influence of Mesenchymal Stem Cell Sources on Their Regenerative Capacities on Different Surfaces. Cells. 2021; 10(2):481. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10020481
Chicago/Turabian StyleMucientes, Arkaitz, Eva Herranz, Enrique Moro, Aranzazu González-Corchón, María Jesús Peña-Soria, Lydia Abasolo, Luis Rodriguez-Rodriguez, Jose Ramon Lamas, and Benjamín Fernández-Gutiérrez. 2021. "Influence of Mesenchymal Stem Cell Sources on Their Regenerative Capacities on Different Surfaces" Cells 10, no. 2: 481. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10020481






