Slack Potassium Channels Modulate TRPA1-Mediated Nociception in Sensory Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Testing
2.2.1. Mechanical Sensitivity
2.2.2. AITC-Induced Pain Behavior
2.2.3. Capsaicin-Induced Pain Behavior
2.3. qRT-PCR
2.4. In Situ Hybridization and Immunostaining
2.5. Ca2+ Imaging
2.6. Patch-Clamp Recordings
2.7. Statistics
3. Results
3.1. Slack−/− Mice Demonstrate Increased Nociceptive Responses to TRPA1 Activation
3.2. TRPA1-Mediated Nociceptive Responses Are Increased in Sensory Neuron-Specific Slack Mutants
3.3. TRPA1-Mediated Nociceptive Responses Are Normal in Spinal Dorsal Horn Neuron-Specific Slack Mutants
3.4. TRPA1-Dependent Calcium Transients in Sensory Neurons Are Unaltered in Slack−/− Mice
3.5. TRPA1 Activation Alters Slack-Mediated Potassium Currents in Sensory Neurons
3.6. TRPA1 Activation Increases Slack-Mediated Potassium Currents In Vitro
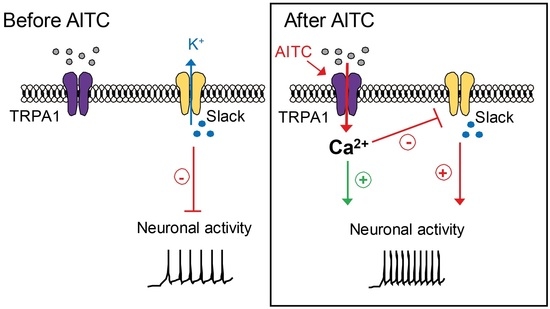
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peirs, C.; Dallel, R.; Todd, A.J. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J. Neural Transm. 2020, 127, 505–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello, R.; Dickenson, A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008, 101, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederberger, E. Novel Insights into Molecular Mechanisms of Chronic Pain. Cells 2020, 9, 2220. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Manion, J.; Waller, M.A.; Clark, T.; Massingham, J.N.; Neely, G.G. Developing Modern Pain Therapies. Front. Neurosci. 2019, 13, 1370. [Google Scholar] [CrossRef]
- Han, Q.; Liu, D.; Convertino, M.; Wang, Z.; Jiang, C.; Kim, Y.H.; Luo, X.; Zhang, X.; Nackley, A.; Dokholyan, N.V.; et al. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 2018, 99, 449–463.e6. [Google Scholar] [CrossRef] [Green Version]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Chu, Y.; Han, L.; Li, M.; Li, Z.; LaVinka, P.C.; Sun, S.; Tang, Z.; Park, K.; Caterina, M.J.; et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014, 81, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, C.; Merlini, J.M.; de Senarclens-Bezencon, C.; Michlig, S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 2020, 10, 11238. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Bamps, D.; Vriens, J.; de Hoon, J.; Voets, T. TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 655–677. [Google Scholar] [CrossRef]
- Akopian, A.N.; Ruparel, N.B.; Patwardhan, A.; Hargreaves, K.M. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008, 28, 1064–1075. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.G.; Faria, R.X. TRPing on the pore phenomenon: What do we know about transient receptor potential ion channel-related pore dilation up to now? J. Bioenerg. Biomembr. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Reese, R.M.; Dourado, M.; Anderson, K.; Warming, S.; Stark, K.L.; Balestrini, A.; Suto, E.; Lee, W.; Riol-Blanco, L.; Shields, S.D.; et al. Behavioral characterization of a CRISPR-generated TRPA1 knockout rat in models of pain, itch, and asthma. Sci. Rep. 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Memon, T.; Chase, K.; Leavitt, L.S.; Olivera, B.M.; Teichert, R.W. TRPA1 expression levels and excitability brake by KV channels influence cold sensitivity of TRPA1-expressing neurons. Neuroscience 2017, 353, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Hogestatt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Zappia, K.J.; O’Hara, C.L.; Moehring, F.; Kwan, K.Y.; Stucky, C.L. Sensory Neuron-Specific Deletion of TRPA1 Results in Mechanical Cutaneous Sensory Deficits. eNeuro 2017, 4, ENEURO.0069-16.2017. [Google Scholar] [CrossRef]
- Lehto, S.G.; Weyer, A.D.; Youngblood, B.D.; Zhang, M.; Yin, R.; Wang, W.; Teffera, Y.; Cooke, M.; Stucky, C.L.; Schenkel, L.; et al. Selective antagonism of TRPA1 produces limited efficacy in models of inflammatory- and neuropathic-induced mechanical hypersensitivity in rats. Mol. Pain 2016, 12, 1744806916677761. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, Y.; Takahashi, K.; Oguma, N.; Tominaga, M.; Ohta, T. Possible involvement of transient receptor potential ankyrin 1 in Ca(2+) signaling via T-type Ca(2+) channel in mouse sensory neurons. J. Neurosci. Res. 2018, 96, 901–910. [Google Scholar] [CrossRef]
- Weng, H.J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.; Wang, Y.; et al. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lin King, J.V.; Paulsen, C.E.; Cheng, Y.; Julius, D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 2020, 585, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lonnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Haring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspani, O.; Zurborg, S.; Labuz, D.; Heppenstall, P.A. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS ONE 2009, 4, e7383. [Google Scholar] [CrossRef] [Green Version]
- Barabas, M.E.; Kossyreva, E.A.; Stucky, C.L. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS ONE 2012, 7, e47988. [Google Scholar] [CrossRef]
- Lu, R.; Bausch, A.E.; Kallenborn-Gerhardt, W.; Stoetzer, C.; Debruin, N.; Ruth, P.; Geisslinger, G.; Leffler, A.; Lukowski, R.; Schmidtko, A. Slack channels expressed in sensory neurons control neuropathic pain in mice. J. Neurosci. 2015, 35, 1125–1135. [Google Scholar] [CrossRef]
- Martinez-Espinosa, P.L.; Wu, J.; Yang, C.; Gonzalez-Perez, V.; Zhou, H.; Liang, H.; Xia, X.M.; Lingle, C.J. Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. Elife 2015, 4, 540. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Metzner, K.; Zhou, F.; Flauaus, C.; Balzulat, A.; Engel, P.; Petersen, J.; Ehinger, R.; Bausch, A.; Ruth, P.; et al. Functional Coupling of Slack Channels and P2X3 Receptors Contributes to Neuropathic Pain Processing. Int. J. Mol. Sci. 2021, 22, 405. [Google Scholar] [CrossRef]
- Evely, K.M.; Pryce, K.D.; Bausch, A.E.; Lukowski, R.; Ruth, P.; Haj-Dahmane, S.; Bhattacharjee, A. Slack KNa Channels Influence Dorsal Horn Synapses and Nociceptive Behavior. Mol. Pain 2017, 13, 1744806917714342. [Google Scholar] [CrossRef] [Green Version]
- Sieber, M.A.; Storm, R.; Martinez-de-la-Torre, M.; Muller, T.; Wende, H.; Reuter, K.; Vasyutina, E.; Birchmeier, C. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J. Neurosci. 2007, 27, 4902–4909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007, 132 (Suppl. S1), S26–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Lu, R.; Flauaus, C.; Kennel, L.; Petersen, J.; Drees, O.; Kallenborn-Gerhardt, W.; Ruth, P.; Lukowski, R.; Schmidtko, A. KCa3.1 channels modulate the processing of noxious chemical stimuli in mice. Neuropharmacology 2017, 125, 386–395. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Metzner, K.; Lu, R.; Petersen, J.; Kuth, M.S.; Heine, S.; Drees, O.; Paul, M.; Becirovic, E.; Kennel, L.; et al. Neuropathic and cAMP-induced pain behavior is ameliorated in mice lacking CNGB1. Neuropharmacology 2020, 171, 108087. [Google Scholar] [CrossRef]
- Schmidtko, A.; Del Turco, D.; Coste, O.; Ehnert, C.; Niederberger, E.; Ruth, P.; Deller, T.; Geisslinger, G.; Tegeder, I. Essential role of the synaptic vesicle protein synapsin II in formalin-induced hyperalgesia and glutamate release in the spinal cord. Pain 2005, 115, 171–181. [Google Scholar] [CrossRef]
- Lu, R.; Lukowski, R.; Sausbier, M.; Zhang, D.D.; Sisignano, M.; Schuh, C.D.; Kuner, R.; Ruth, P.; Geisslinger, G.; Schmidtko, A. BKCa channels expressed in sensory neurons modulate inflammatory pain in mice. Pain 2014, 155, 556–565. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Li, Y.; Liang, X.; Sun, Q.; Yu, H.; Zhong, J.; Ni, Y.; Chen, J.; Zhao, Z.; et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc. Diabetol. 2015, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, N.; Offermanns, S.; Kuner, R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 2004, 38, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Haring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lonnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Gao, W.; Konig, P.; Heine, S.; Motterlini, R.; Ruth, P.; Schlossmann, J.; Koesling, D.; Niederberger, E.; Tegeder, I.; et al. cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I. J. Neurosci. 2008, 28, 8568–8576. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, H.; Pawlowski, S.A.; Fraine, S.L.; Sharif, B.; Hamad, D.; Fatima, T.; Berg, J.; Brown, C.M.; Jan, L.Y.; Ribeiro-da-Silva, A.; et al. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015, 13, 1246–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 2007, 10, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Sura, L.; Benedikt, J.; Ettrich, R.; Minofar, B.; Teisinger, J.; Vlachova, V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem. J. 2011, 433, 197–204. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflugers Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef]
- Bobkov, Y.V.; Corey, E.A.; Ache, B.W. The pore properties of human nociceptor channel TRPA1 evaluated in single channel recordings. Biochim. Biophys. Acta 2011, 1808, 1120–1128. [Google Scholar] [CrossRef] [Green Version]
- Budelli, G.; Sun, Q.; Ferreira, J.; Butler, A.; Santi, C.M.; Salkoff, L. SLO2 Channels Are Inhibited by All Divalent Cations That Activate SLO1 K+ Channels. J. Biol. Chem. 2016, 291, 7347–7356. [Google Scholar] [CrossRef] [Green Version]
- Patton, C.; Thompson, S.; Epel, D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004, 35, 427–431. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, J.Y.; Kim, T.H.; Paik, S.K.; Dai, Y.; Noguchi, K.; Ahn, D.K.; Bae, Y.C. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J. Comp. Neurol. 2010, 518, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Chesler, A.T.; Braz, J.M.; Shah, N.M.; Julius, D.; Basbaum, A.I. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 2011, 31, 10119–10127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Suzuki, Y.; Uchida, K.; Tominaga, M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat. Commun. 2013, 4, 2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmiedl, S.; Peters, D.; Schmalz, O.; Mielke, A.; Rossmanith, T.; Diop, S.; Piefke, M.; Thurmann, P.; Schmidtko, A. Loxapine for Treatment of Patients With Refractory, Chemotherapy-Induced Neuropathic Pain: A Prematurely Terminated Pilot Study Showing Efficacy But Limited Tolerability. Front. Pharmacol. 2019, 10, 838. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.Y.; Xu, H.; Clapham, D.E.; Ji, R.R. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J. Neurosci. 2004, 24, 8300–8309. [Google Scholar] [CrossRef] [Green Version]
- Braz, J.M.; Basbaum, A.I. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain 2010, 150, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Ehinger, R.; Kuret, A.; Matt, L.; Frank, N.; Wild, K.; Kabagema-Bilan, C.; Bischof, H.; Malli, R.; Ruth, P.; Bausch, A.E.; et al. Slack K(+) channels attenuate NMDA-induced excitotoxic brain damage and neuronal cell death. FASEB J. 2021, 35, e21568. [Google Scholar] [CrossRef]
- Joiner, W.J.; Tang, M.D.; Wang, L.Y.; Dworetzky, S.I.; Boissard, C.G.; Gan, L.; Gribkoff, V.K.; Kaczmarek, L.K. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat. Neurosci. 1998, 1, 462–469. [Google Scholar] [CrossRef]
- Santi, C.M.; Ferreira, G.; Yang, B.; Gazula, V.R.; Butler, A.; Wei, A.; Kaczmarek, L.K.; Salkoff, L. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J. Neurosci. 2006, 26, 5059–5068. [Google Scholar] [CrossRef] [Green Version]
- Barcia, G.; Fleming, M.R.; Deligniere, A.; Gazula, V.R.; Brown, M.R.; Langouet, M.; Chen, H.; Kronengold, J.; Abhyankar, A.; Cilio, R.; et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 2012, 44, 1255–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, X.; Ostertag, E.M.; Nuwal, T.; Huang, B.; Jan, Y.N.; Basbaum, A.I.; Jan, L.Y. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 2013, 16, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.R.; Kaczmarek, L.K. Use of optical biosensors to detect modulation of Slack potassium channels by G protein-coupled receptors. J. Recept. Signal Transduct Res. 2009, 29, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, L.K. Slack, Slick and Sodium-Activated Potassium Channels. ISRN Neurosci. 2013, 2013, 354262. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, L.K.; Aldrich, R.W.; Chandy, K.G.; Grissmer, S.; Wei, A.D.; Wulff, H. International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels. Pharmacol. Rev. 2017, 69, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Brown, M.R.; Hyland, C.; Chen, Y.; Kronengold, J.; Fleming, M.R.; Kohn, A.B.; Moroz, L.L.; Kaczmarek, L.K. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. J. Neurosci. 2012, 32, 15318–15327. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Metzner, K.; Engel, P.; Balzulat, A.; Sisignano, M.; Ruth, P.; Lukowski, R.; Schmidtko, A.; Lu, R. Slack Potassium Channels Modulate TRPA1-Mediated Nociception in Sensory Neurons. Cells 2022, 11, 1693. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11101693
Zhou F, Metzner K, Engel P, Balzulat A, Sisignano M, Ruth P, Lukowski R, Schmidtko A, Lu R. Slack Potassium Channels Modulate TRPA1-Mediated Nociception in Sensory Neurons. Cells. 2022; 11(10):1693. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11101693
Chicago/Turabian StyleZhou, Fangyuan, Katharina Metzner, Patrick Engel, Annika Balzulat, Marco Sisignano, Peter Ruth, Robert Lukowski, Achim Schmidtko, and Ruirui Lu. 2022. "Slack Potassium Channels Modulate TRPA1-Mediated Nociception in Sensory Neurons" Cells 11, no. 10: 1693. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11101693