Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights
Abstract
:1. Introduction
Breast Cancer Stem Cells
2. Drug Repurposing: The Anti-Diabetic to Anticancer Action of Metformin
3. AMPK Activation
Mechanism of AMPK Activation
4. AMPK Activation for the Treatment of Breast Cancer Stem Cells
4.1. Activated AMPK Downregulates Cyclin Proteins and Induces Cell Cycle Arrest and Autophagy
4.2. AMPK Activation Inhibits Lipogenic Enzymes
4.3. AMPK Activation Downregulates the Mammalian Target of the Rapamycin (mTOR) Pathway and Insulin Growth Factors (IGFs)
4.4. AMPK Activity Opposes the Warburg Effect
4.5. Anticancer Stem Cell Action of AMPK
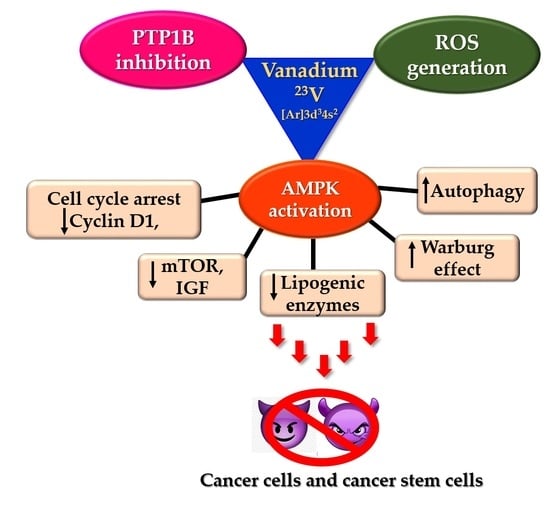
5. Vanadium Compounds: Insulin Mimetic Agents with Possible Anticancer Action
5.1. Anti-Diabetic Action of Vanadium—PTP1B Inhibition
5.2. PTP1B and Breast Cancer
5.3. PTP1B Inhibition of AMPK Activation: Can Vanadium Compounds Activate AMPK?
5.4. Vanadium-Mediated AMPK Activation—Reduced Adipogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 22 November 2021).
- Dittmer, J. Breast cancer stem cells: Features, key drivers and treatment options. Semin. Cancer Biol. 2018, 53, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butti, R.; Gunasekaran, V.P.; Kumar, T.V.S.; Banerjee, P.; Kundu, G.C. Breast cancer stem cells: Biology and therapeutic implications. Int. J. Biochem. Cell Biol. 2019, 107, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chen, Q.; Zou, Y.; Chen, H.; Qi, L.; Chen, Y. Stem cells and cellular origins of breast cancer: Updates in the rationale, controversies, and therapeutic implications. Front. Oncol. 2019, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Ponti, D.; Zaffaroni, N.; Capelli, C.; Daidone, M.G. Breast cancer stem cells: An overview. Eur. J. Cancer 2006, 42, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Patel, M.R.; Prescher, J.A.; Patsialou, A.; Qian, D.; Lin, J.; Wen, S.; Chang, Y.F.; Bachmann, M.H.; Shimono, Y.; et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc. Natl. Acad. Sci. USA 2010, 107, 18115–18120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting breast cancer stem cells to overcome treatment resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wicha, M.S. Targeting breast cancer stem cells. J. Clin. Oncol. 2010, 28, 4006–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Castillo, B.; Lopez-Bonet, E.; Cuyàs, E.; Viñas, G.; Pernas, S.; Dorca, J.; Menendez, J.A. Cancer stem cell-driven efficacy of trastuzumab (Herceptin): Towards a reclassification of clinically HER2-positive breast carcinomas. Oncotarget 2015, 6, 32317–32338. [Google Scholar] [CrossRef]
- Korkaya, H.; Paulson, A.; Iovino, F.; Wicha, M.S. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008, 27, 6120–6130. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Jin, W.; Zhang, J.; Zhu, L.; Lu, J.; Zhen, Y.; Zhang, L.; Ouyang, L.; Liu, B.; Yu, H. Repurposing non-oncology small-molecule drugs to improve cancer therapy: Current situation and future directions. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Waissengrin, B.; Wolf, I.; Zahavi, T.; Salmon-Divon, M.; Sonnenblick, A. The effect of non-oncology drugs on clinical and genomic risk in early luminal breast cancer. J. Clin. Oncol. 2021, 39, e12505. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, J.; Liu, H. Drug repurposing in oncology: Current evidence and future direction. Curr. Med. Chem. 2021, 28, 2175–2194. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anticancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beijersbergen, R.L. Old drugs with new tricks. Nat. Cancer 2020, 1, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, R.J.O.; Goodwin, P.J.; Stambolic, V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [CrossRef]
- Gillies, R.J.; Pilot, C.; Marunaka, Y.; Fais, S. Targeting acidity in cancer and diabetes. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sahra, I.B.; Regazzetti, C.; Robert, G.; Laurent, K.; le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.L.; Gans, R.O.B.; Vries, E.G.E.D. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wu, W.; Yang, F.; Liu, L.; Yang, Z.; Liu, L.; Tang, W.; Sun, F.; Lin, H. Marine sponge-derived smenospongine preferentially eliminates breast cancer stem-like cells via p38/AMPKα pathways. Cancer Med. 2018, 7, 3965–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Orhan, Y.C.; Zha, X.; Esencan, E.; Chatterton, R.T.; Bulun, S.E. AMP-activated protein kinase and energy balance in breast cancer. Am. J. Transl. Res. 2017, 9, 197–213. [Google Scholar]
- Song, C.W.; Lee, H.; Dings, R.P.M.; Williams, B.; Powers, J.; dos Santos, T.; Choi, B.H.; Park, H.J. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci. Rep. 2012, 2, 362. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Pulinilkunnil, T.; Murano, I.; Bence, K.K.; He, H.; Minokoshi, Y.; Asakura, K.; Lee, A.; Haj, F.; Furukawa, N.; et al. Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol. Cell. Biol. 2009, 29, 4563–4573. [Google Scholar] [CrossRef] [Green Version]
- Pinter, K.; Jefferson, A.; Czibik, G.; Watkins, H.; Redwood, C. Subunit composition of AMPK trimers present in the cytokinetic apparatus: Implications for drug target identification. Cell Cycle 2012, 11, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.J.; Li, J.; Russell, R.R.; Neumann, D.; Miller, E.J.; Tuerk, R.; Wallimann, T.; Hurley, R.L.; Witters, L.A.; Young, L.H. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ. Res. 2005, 96, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, W.; Wu, F.; Wang, C.; Yu, L.; Tang, L.; Qiu, B.; Li, Y.; Guo, L.; Wu, M.; et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 5372–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.Y.; He, Y.Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front. Oncol. 2013, 3, 175. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-C.; Chou, C.-C.; Kulp, S.K.; Chen, C.-S. AMPK as a potential anticancer target—Friend or foe? Curr. Pharm. Des. 2014, 20, 2607–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhaji-Trajkovic, L.; Vilimanovich, U.; Kravic-Stevovic, T.; Bumbasirevic, V.; Trajkovic, V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J. Cell. Mol. Med. 2009, 13, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and cancer. In AMP-activated Protein Kinase; Cordero, M.D., Viollet, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 203–226. ISBN 978-3-319-43589-3. [Google Scholar]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9, 190099. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Chen, D.; Ao, J.; Zhang, W.; Yi, J.; Ren, X.; Fei, J.; Li, F.; Niu, M.; Chen, H.; et al. Transcriptional suppression of AMPKα1 promotes breast cancer metastasis upon oncogene activation. Proc. Natl. Acad. Sci. USA 2020, 117, 8013–8021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Zou, H.; Xiao, T.; Liu, X.; Wang, Q.; Cheng, J.; Fu, S.; Peng, J.; Xie, X.; Fu, J. TQFL12, a novel synthetic derivative of TQ, inhibits triple-negative breast cancer metastasis and invasion through activating AMPK/ACC pathway. J. Cell Mol. Med. 2021, 25, 10101–10110. [Google Scholar] [CrossRef] [PubMed]
- Penugurti, V.; Khumukcham, S.S.; Padala, C.; Dwivedi, A.; Kamireddy, K.R.; Mukta, S.; Bhopal, T.; Manavathi, B. HPIP protooncogene differentially regulates metabolic adaptation and cell fate in breast cancer cells under glucose stress via AMPK and RNF2 dependent pathways. Cancer Lett. 2021, 518, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Hoang, D.H.; Song, M.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Melatonin and doxorubicin synergistically enhance apoptosis via autophagy-dependent reduction of AMPKα1 transcription in human breast cancer cells. Exp Mol Med. 2021, 53, 1413–1422. [Google Scholar] [CrossRef]
- Seto-Tetsuo, F.; Arioka, M.; Miura, K.; Inoue, T.; Igawa, K.; Tomooka, K.; Takahashi-Yanaga, F.; Sasaguri, T. DIF-1 inhibits growth and metastasis of triple-negative breast cancer through AMPK-mediated inhibition of the mTORC1-S6K signaling pathway. Oncogene 2021, 40, 5579–5589. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, H.I.; Kim, S.J.; Park, S.H.; Kim, H.S. Paclitaxel suppresses the viability of breast tumor MCF7 cells through the regulation of EF1á and FOXO3a by AMPK signaling. Int. J. Oncol. 2015, 47, 1874–1880. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Li, J.; Hao, Q.; Vadgama, J.V.; Wu, Y. AMP-activated protein kinase: A potential therapeutic target for triple-negative breast cancer 11 medical and health sciences 1112 oncology and carcinogenesis. Breast Cancer Res. 2019, 21, 29. [Google Scholar] [CrossRef] [Green Version]
- Zadra, G.; Batista, J.L.; Loda, M. Dissecting the dual role of AMPK in cancer: From experimental to human studies. Mol. Cancer Res. 2015, 13, 1059–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.C.; Lee, K.H.; Lai, I.L.; Wang, D.; Mo, X.; Kulp, S.K.; Shapiro, C.L.; Chen, C.S. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014, 74, 4783–4795. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Hsu, E.C.; Guh, J.H.; Yang, H.C.; Wang, D.; Kulp, S.K.; Shapiro, C.L.; Chen, C.S. Targeting energy metabolic and oncogenic signaling pathways in triple-negative breast cancer by a novel adenosine monophosphate-activated protein kinase (AMPK) activator. J. Biol. Chem. 2011, 286, 39247–39258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Storr, S.J.; Johnson, K.; Green, A.R.; Rakha, E.A.; Ellis, I.O.; Morgan, D.A.L.; Martin, S.G. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget 2014, 5, 12936–12949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sante, G.; Page, J.; Jiao, X.; Nawab, O.; Cristofanilli, M.; Skordalakes, E.; Pestell, R.G. Recent advances with cyclin-dependent kinase inhibitors: Therapeutic agents for breast cancer and their role in immuno- oncology. Expert Rev. Anticancer Ther. 2019, 19, 569–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, G.; Dai, M.; Zhang, C.; Poulet, S.; Moamer, A.; Wang, N.; Boudreault, J.; Ali, S.; Lebrun, J.J. TGFβ/cyclin D1/Smad-mediated inhibition of BMP4 promotes breast cancer stem cell self-renewal activity. Oncogenesis 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Al-Odaini, A.A.; Fils-Aimé, N.; Villatoro, M.A.; Guo, J.; Arakelian, A.; Rabbani, S.A.; Ali, S.; Lebrun, J.J. Erratum to: Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2017, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- Millar, E.K.A.; Dean, J.L.; McNeil, C.M.; O’Toole, S.A.; Henshall, S.M.; Tran, T.; Lin, J.; Quong, A.; Comstock, C.E.S.; Witkiewicz, A.; et al. Cyclin D1b protein expression in breast cancer is independent of cyclin D1a and associated with poor disease outcome. Oncogene 2009, 28, 1812–1820. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Huang, L.; Ye, F.; Shi, M.F.; Cheng, X.D.; Wang, X.Y.; Hu, D.X.; Xie, X.; Lu, W.G. Cyclin D1 affects epithelial-mesenchymal transition in epithelial ovarian cancer stem cell-like cells. OncoTargets Ther. 2013, 6, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.; Rychahou, P.; Sviripa, V.M.; Weiss, H.L.; Liu, C.; Watt, D.S.; Evers, B.M. Induction of AMPK activation by N,N′-diarylurea FND-4b decreases growth and increases apoptosis in triple negative and estrogen-receptor positive breast cancers. PLoS ONE 2019, 14, e0209392. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Keith, W.K. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J. Mol. Signal. 2008, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Yu, L.F.; Nan, F.J.; Feng, L.Y.; Li, J. AMP-activated protein kinase is involved in neural stem cell growth suppression and cell cycle arrest by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and glucose deprivation by down-regulating phospho-retinoblastoma protein and cyclin D. J. Biol. Chem. 2009, 284, 6175–6184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Sheng, H.; Zhang, X.; Qi, Q.; Chan, C.B.; Li, L.; Shan, C.; Ye, K. Cellular energy stress induces AMPK-mediated regulation of glioblastoma cell proliferation by PIKE-A phosphorylation. Cell Death Dis. 2019, 10, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Shao, S.H.; Xu, Z.-X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1–AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef]
- Casimiro, M.C.; di Sante, G.; di Rocco, A.; Loro, E.; Pupo, C.; Pestell, T.G.; Bisetto, S.; Velasco-Velázquez, M.A.; Jiao, X.; Li, Z.; et al. Cyclin D1 restrains oncogene-induced autophagy by regulating the AMPK–LKB1 signaling axis. Cancer Res. 2017, 77, 3391–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohmen, M.; Krieg, S.; Agalaridis, G.; Zhu, X.; Shehata, S.N.; Pfeiffenberger, E.; Amelang, J.; Bütepage, M.; Buerova, E.; Pfaff, C.M.; et al. AMPK-dependent activation of the Cyclin Y/CDK16 complex controls autophagy. Nat. Commun. 2020, 11, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryal, P.; Kim, K.; Park, P.H.; Ham, S.; Cho, J.; Song, K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, D.; Gao, S.; Gao, Y.; Ye, J.; Liu, P. Cyclovirobuxine D induces autophagy-associated cell death via the Akt/mTOR pathway in MCF-7 human breast cancer cells. J. Pharmacol. Sci. 2014, 125, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Hu, X.; Tan, X.; Cheng, W.; Wang, Q.; Chen, X.; Guan, Y.; Chen, C.; Jing, X. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of Esophageal squamous cell carcinomas in vitro and in vivo. PLoS ONE 2015, 10, e0133349. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.T.; Ho, H.J.; Lin, J.T.; Shieh, J.J.; Wu, C.Y. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017, 8, e2626. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, E.A.I.F.; Puukila, S.; Eichler, R.; Sampaio, S.C.; Forsyth, H.L.; Lees, S.J.; Barbosa, A.M.; Dekker, R.F.H.; Fortes, Z.B.; Khaper, N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS ONE 2014, 9, e98207. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, L.; Liu, C.; Xia, T.; Zha, X.; Wang, S. Phenformin induces cell cycle change, apoptosis, and mesenchymal-epithelial transition and regulates the AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells. PLoS ONE 2015, 10, e0131207. [Google Scholar] [CrossRef]
- Cai, J.; Qiong, G.; Li, C.; Sun, L.; Luo, Y.; Yuan, S.; Gonzalez, F.J.; Xu, J. Manassantin B attenuates obesity by inhibiting adipogenesis and lipogenesis in an AMPK dependent manner. FASEB J. 2021, 35, e21496. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, Y.; Zheng, M.; Yin, L.; Wang, X.; Fu, Y.; Yu, B.; Li, J. Polyphenols from blue honeysuckle (Lonicera caerulea var. edulis) berry inhibit lipid accumulation in adipocytes by suppressing lipogenesis. J. Ethnopharmacol. 2021, 279, 114403. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, G.; Hu, T.; Mo, X.; Hou, X.; Cao, K.; Wang, L.; Pan, Z.; Wu, Q.; Li, X.; et al. Resveratrol ameliorates lipid accumulation and inflammation in human SZ95 sebocytes via the AMPK signaling pathways in vitro. J. Dermatol. Sci. 2021, 103, 156–166. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.W.; Corl, B.A. Activation of AMP-activated protein kinase (AMPK) inhibits fatty acid synthesis in bovine mammary epithelial cells. Biochem. Biophys. Res. Commun. 2009, 390, 388–393. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schul, A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Guo, D.; Hildebrandt, I.J.; Prins, R.M.; Soto, H.; Mazzotta, M.M.; Dang, J.; Czernin, J.; Shyy, J.Y.J.; Watson, A.D.; Phelps, M.; et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12932–12937. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-H.; Tsai, S.-J.; Wang, Y.-J.; Pan, M.-H.; Kao, J.-Y.; Way, T.-D. EGCG inhibits protein synthesis, lipogenesis, andcell cycle progression through activation of AMPK inp53 positive and negative human hepatoma cells. Mol. Nutr. Food Res. 2009, 53, 1156–1165. [Google Scholar] [CrossRef]
- Zadra, G.; Priolo, C.; Patnaik, A.; Loda, M. New strategies in prostate cancer: Targeting lipogenic pathways and the energy sensor AMPK. Clin. Cancer Res. 2010, 16, 3322–3328. [Google Scholar] [CrossRef] [Green Version]
- Zadra, G.; Photopoulos, C.; Tyekucheva, S.; Heidari, P.; Weng, Q.P.; Fedele, G.; Liu, H.; Scaglia, N.; Priolo, C.; Sicinska, E.; et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol. Med. 2014, 6, 519–538. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, H.; Chen, D.; Guo, D.; Wang, X. Targeting at cancer energy metabolism and lipid droplet formation as new treatment strategies for epigallocatechin-3-gallate (EGCG) in colorectal cancer cells. J. Funct. Foods 2021, 83, 104570. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Pamu, S.; Cui, Q.; Chan, T.H.; Dou, Q.P. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorgan. Med. Chem. 2012, 20, 3031–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, S.; Favre, C.; Lasorsa, F.M.; Leahy, M.; Trigiante, G.; Stroebel, P.; Marx, A.; Loughran, G.; O’Callaghan, K.; Marobbio, C.M.T.; et al. The insulin-like growth factor-I–mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol. Biol. Cell 2007, 18, 3545–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar]
- Francipane, M.G.; Lagasse, E. Therapeutic potential of mTOR inhibitors for targeting cancer stem cells. Br. J. Clin. Pharmacol. 2016, 82, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Min, Y. Pre-clinical evidence that salinomycin is active against retinoblastoma via inducing mitochondrial dysfunction, oxidative damage and AMPK activation. J. Bioenerg. Biomembr. 2021, 53, 513–523. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival ken. Cells 2003, 115, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, H.J.; Park, C.S.; Oh, E.T.; Choi, B.H.; Williams, B.; Lee, C.K.; Song, C.W. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS ONE 2014, 9, e87979. [Google Scholar] [CrossRef]
- Kawakita, E.; Yang, F.; Kumagai, A.; Takagaki, Y.; Kitada, M.; Yoshitomi, Y.; Ikeda, T.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; et al. Metformin mitigates DPP-4 inhibitor-induced breast cancer metastasis via suppression of mTOR signaling. Mol. Cancer Res. 2020, 16, 61–73. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. Correction to: ‘The Warburg EFFECT: How does it benefit cancer cells?’. Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK is a negative regulator of the Warburg EFFECT and suppresses tumor growth in vivo opposes tumor development, and its loss fosters tumor progression in part by regulating cellular metabolic pathways that support cell growth and proliferation. Cell Metab. Jan. 2013, 8, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wong, C.C.; Zhang, X.; Kang, W.; Nakatsu, G.; Zhao, Q.; Chen, H.; Go, M.Y.Y.; Chiu, P.W.Y.; Wang, X.; et al. CAB39L elicited an anti-Warburg effect via a LKB1-AMPK-PGC1α axis to inhibit gastric tumorigenesis. Oncogene 2018, 37, 6383–6398. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Zhu, Y.J.; Hu, C.H.; You, L.; Wu, J.; He, X.Y.; Huang, W.J.; Wu, Z.H. Ghrelin affects gastric cancer progression by activating AMPK signaling pathway. Biochem. Genet. 2021, 59, 652–667. [Google Scholar] [CrossRef]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in lps-stimulated macrophages and endotoxin-shocked mice through an ampk/akt/nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Choi, S.S.; Park, M.H.; Jang, H.; Lee, Y.H.; Khim, K.W.; Oh, S.R.; Park, J.; Ryu, H.W.; Choi, J.H. Broussonetia papyrifera root bark extract exhibits anti-inflammatory effects on adipose tissue and improves insulin sensitivity potentially via AMPK activation. Nutrients 2020, 12, 773. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer stem cells behavior. Stem Cell Res. Ther. 2021, 12, 245–255. [Google Scholar] [CrossRef]
- Li, Y.H.; Luo, J.; Mosley, Y.Y.C.; Hedrick, V.E.; Paul, L.N.; Chang, J.; Zhang, G.; Wang, Y.K.; Banko, M.R.; Brunet, A.; et al. AMP-Activated protein kinase directly phosphorylates and destabilizes Hedgehog pathway transcription factor GLI1 in medulloblastoma. Cell Rep. 2015, 12, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Kwan, H.T.; Chan, D.W.; Cai, P.C.H.; Mak, C.S.L.; Yung, M.M.H.; Leung, T.H.Y.; Wong, O.G.W.; Cheung, A.N.Y.; Ngan, H.Y.S. AMPK activators suppress cervical cancer cell growth through Inhibition of DVL3 mediated Wnt/b-catenin signaling activity. PLoS ONE 2013, 8, e53597. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.F.; Xie, C.W.; Yang, S.X.; Xiong, J.P. AMPK activators suppress breast cancer cell growth by inhibiting DVL3-facilitated Wnt/β-catenin signaling pathway activity. Mol. Med. Rep. 2017, 15, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Bao, B.; Azmi, A.S.; Ali, S.; Zaiem, F.; Sarkar, F.H. Metformin may function as anti-cancer agent via targeting cancer stem cells: The potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann. Transl. Med. 2014, 2, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Dronamraju, V.; Ibrahim, B.A.; Briski, K.P.; Sylvester, P.W. γ-tocotrienol suppression of the warburg effect is mediated by AMPK activation in human breast cancer cells. Nutr. Cancer 2019, 71, 1214–1228. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.B.; Zhang, X.Y.; Lin, Q.R.; Xu, L.H.; Zhao, G.X.; Pan, H.; Zhou, D.; Ouyang, D.Y.; Liu, Z.H.; He, X.H. Cucurbitacin e induces autophagy via downregulating mTORC1 signaling and upregulating AMPK activity. PLoS ONE 2015, 10, e0124355. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Maxim, C.; Manduteanu, I.; Calin, M.; Popescu, D.L. Synthesis, characterization, and in vitro insulin-mimetic activity evaluation of valine schiff base coordination compounds of oxidovanadium(v). Biomedicines 2021, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Mordyl, B.; Menaszek, E.; Sapa, J. Characterization and antidiabetic activity of salicylhydrazone Schiff base vanadium(IV) and (V) complexes. Transit. Met. Chem. 2021, 46, 201–217. [Google Scholar] [CrossRef]
- Suman, S.G.; Gretarsdottir, J.M. Chemical and clinical aspects of metal-containing antidotes for poisoning by cyanide. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver, P.L., Ed.; De Gruyter: Berlin, Germany, 2019; pp. 359–392. [Google Scholar]
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from phase 0 to phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef]
- Crans, D.C. Antidiabetic, chemical, and physical properties of organic vanadates as presumed transition-state inhibitors for phosphatases. J. Org. Chem. 2015, 80, 11899–11915. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Sakurai, H.; Kojima, Y.; Yoshikawa, Y.; Kawabe, K.; Yasui, H. Antidiabetic vanadium(IV) and zinc(II) complexes. Coord. Chem. Rev. 2002, 226, 187–198. [Google Scholar] [CrossRef]
- Kawabe, K.; Yoshikawa, Y.; Adachi, Y.; Sakurai, H. Possible mode of action for insulinomimetic activity of vanadyl(IV) compounds in adipocytes. Life Sci. 2006, 78, 2860–2866. [Google Scholar] [CrossRef]
- Irving, E.; Stoker, A.W. Vanadium compounds as PTP inhibitors. Molecules 2017, 22, 2269. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, C.; Vilter, H.; Fournier, J.B.; Delage, L.; Potin, P.; Rebuffet, E.; Michel, G.; Solari, P.L.; Feiters, M.C.; Czjzek, M. Vanadium haloperoxidases: From the discovery 30 years ago to X-ray crystallographic and V K-edge absorption spectroscopic studies. Coord. Chem. Rev. 2015, 301–302, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Crans, D.C. Fifteen years of dancing with vanadium. Pure Appl. Chem. 2005, 77, 1497–1527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Y. Protein-tyrosine phosphatases: Biological function, structural characteristics, and mechanism of catalysis. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-X.; Zhang, Z.-Y. Targeting PTPs with small molecule inhibitors in cancer treatment. Cancer Metastasis Rev. 2008, 27, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in biological action: Chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilmarsdottir, B.; Briem, E.; Halldorsson, S.; Kricker, J.; Ingthorsson, S.; Gustafsdottir, S.; Mælandsmo, G.M.; Magnusson, M.K.; Gudjonsson, T. Inhibition of PTP1B disrupts cell—Cell adhesion and induces anoikis in breast epithelial cells. Cell Death Dis. 2017, 8, e2769. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.N.N.; Lyra e Silva, N.M.; Ferreira, S.T.; de Felice, F.G. Protein tyrosine phosphatase 1B (PTP1B): A potential target for Alzheimer’s therapy? Front. Aging Neurosci. 2017, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Xiao, R.; Wang, N.; Wang, Z.; Zhang, Y.; Xia, Q.; Yang, X. The molecular mechanisms and rational design of anti-diabetic vanadium compounds. Curr. Top. Med. Chem. 2016, 16, 811–822. [Google Scholar] [CrossRef]
- Tamrakar, A.K.; Maurya, C.K.; Rai, A.K. PTP1B inhibitors for type 2 diabetes treatment: A patent review (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1101–1115. [Google Scholar] [CrossRef]
- Yang, J.L.; Ha, T.K.Q.; Lee, B.W.; Kim, J.; Oh, W.K. PTP1B inhibitors from the seeds of Iris sanguinea and their insulin mimetic activities via AMPK and ACC phosphorylation. Bioorgan. Med. Chem. Lett. 2017, 27, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Beyene, Z. The protein tyrosine phosphatase PTB1B role in the development of obesity, diabetes, and cancer and its potential inhibitors. Int. J. Pharm. Bio-Med. Sci. 2021, 1, 88–101. [Google Scholar] [CrossRef]
- Soysal, S.; Obermann, E.C.; Gao, F.; Oertli, D.; Gillanders, W.E.; Viehl, C.T.; Muenst, S. PTP1B expression is an independent positive prognostic factor in human breast cancer. Breast Cancer Res. Treat. 2013, 137, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Pan, Y.; Zhao, L.; Qi, F.; Liu, J. Protein tyrosine phosphatase 1B(PTP1B) promotes melanoma cells progression through Src activation. Bioengineered 2021, 12, 8396–8406. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K.; Muthuswamy, S.K. A brake becomes an accelerator: PTP1B-A new therapeutic target for breast cancer. Cancer Cell 2007, 11, 214–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentires-Alj, M.; Neel, B.G. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007, 67, 2420–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, N.; Koveal, D.; Miller, D.H.; Xue, B.; Akshinthala, S.D.; Kragelj, J.; Jensen, M.R.; Gauss, C.-M.; Page, R.; Blackledge, M.; et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 2014, 10, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Wiener, J.R.; Kerns, B.-J.M.; Harvey, E.L.; Conaway, M.R.; Lglehart, J.D.; Berchuck, A.; Bast, R.C., Jr. Overexpression of the protien tyrosine phosphatase PTP1B in human breast cancer: Assocation with p185 c-erbB-2 protein expression. J. Natl. Cancer Inst. 1994, 86, 372–378. [Google Scholar] [CrossRef]
- Liao, S.C.; Li, J.X.; Yu, L.; Sun, S.R. Protein tyrosine phosphatase 1B expression contributes to the development of breast cancer. J. Zhejiang Univ. Sci. B 2017, 18, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zeng, Q.; Qiu, J.; Pang, T.; Xian, J.; Zhang, X. Long non-coding RNA UCA1 promotes breast cancer by upregulating PTP1B expression via inhibiting miR-206. Cancer Cell Int. 2019, 19, 275. [Google Scholar] [CrossRef]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; lo Bosco, G.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B phosphatase as a novel target of oleuropein activity in MCF-7 breast cancer model. Toxicol. Vitr. 2019, 61, 104624. [Google Scholar] [CrossRef] [PubMed]
- Arregui, C.O.; González, Á.; Burdisso, J.E.; Wusener, A.E.G. Protein tyrosine phosphatase PTP1B in cell adhesion and migration. Cell Adhes. Migr. 2013, 7, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.A.; Biggins, L.; Sharpe, H.J. Protein tyrosine phosphatases in cell adhesion. Biochem. J. 2021, 478, 1061–1083. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, T.; Wołosewicz, K.; Jamrozik, M.; Drzeżdżon, J.; Siemińska, J.; Jacewicz, D.; Górska-Ponikowska, M.; Kołaczkowski, M.; Łaźny, R.; Kuban-Jankowska, A. Curcumin and its new derivatives: Correlation between cytotoxicity against breast cancer cell lines, degradation of PTP1B phosphatase and ROS generation. Int. J. Mol. Sci. 2021, 22, 10368. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, G.; Mu, Y.; Wu, W.; Cao, B.; Wang, Z.; Yu, H.; Guan, P.; Han, L.; Li, L.; et al. Discovery of anti-TNBC agents targeting PTP1B: Total synthesis, structure-activity relationship, in vitro and in vivo investigations of jamunones. J. Med. Chem. 2021, 64, 6008–6020. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Kostrzewa, T.; Musial, C.; Barone, G.; Lo-Bosco, G.; Lo-Celso, F.; Gorska-Ponikowska, M. Green tea catechins induce inhibition of ptp1b phosphatase in breast cancer cells with potent anti-cancer properties: In vitro assay, molecular docking, and dynamics studies. Antioxidants 2020, 9, 1208. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Gorska-Ponikowska, M.; Wozniak, M. Lipoic acid decreases the viability of breast cancer cells and activity of PTP1B and SHP2. Anticancer Res. 2017, 37, 2893–2898. [Google Scholar] [CrossRef] [Green Version]
- To, D.C.; Hoang, D.T.; Tran, M.H.; Pham, M.Q.; Huynh, N.T.; Nguyen, P.H. PTP1B inhibitory flavonoids from orthosiphon stamineus benth. and their growth inhibition on human breast cancer cells. Nat. Prod. Commun. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Gorska-Ponikowska, M.; Sahu, K.K. Docosahexaenoic acid inhibits PTP1B phosphatase. Nutrients 2019, 11, 2554. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Sharma, G.; Yang, J.-L.; Choi, H.S.; Lim, S.-I.; Kang, K.W.; Oh, W.K. Oleanane triterpenes as protein tyrosine phosphatase 1B (PTP1B) inhibitors from Camellia japonica. Phytochemistry 2014, 103, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, T.; Przychodzen, P.; Gorska-Ponikowska, M.; Kuban-Jankowska, A. Curcumin and cinnamaldehyde as PTP1B inhibitors with antidiabetic and anticancer potential. Anticancer Res. 2019, 39, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Sharma, G.; Dao, T.T.; Uddin, M.N.; Kang, K.W.; Ndinteh, D.T.; Mbafor, J.T.; Oh, W.K. New prenylated isoflavonoids as protein tyrosine phosphatase 1B (PTP1B) inhibitors from Erythrina addisoniae. Bioorgan. Med. Chem. 2012, 20, 6459–6464. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Le, T.V.T.; Thuong, P.T.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Kang, K.W.; Oh, W.K. Cytotoxic and PTP1B inhibitory activities from Erythrina abyssinica. Bioorgan. Med. Chem. Lett. 2009, 19, 6745–6749. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Sahu, K.K.; Gorska-Ponikowska, M.; Tuszynski, J.A.; Wozniak, M. Inhibitory activity of iron chelators ATA and DFO on MCF-7 breast cancer cells and phosphatases PTP1B and SHP2. Anticancer Res. 2017, 37, 4799–4806. [Google Scholar]
- Chandel, S.; Manikandan, A.; Mehta, N.; Nathan, A.A.; Tiwari, R.K.; Mohapatra, S.B.; Chandran, M.; Jaleel, A.; Manoj, N.; Dixit, M. Protein tyrosine phosphatase-PEST (PTP-PEST) mediates hypoxia-induced endothelial autophagy and angiogenesis through AMPK activation. J. Cell Sci. 2020, 134, jcs250274. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, N.; Li, X.; Guo, C.; Li, C.; Jiang, B.; Wang, H.; Shi, D. Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death Dis. 2019, 10, 874. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lee, Y.J.; Jin, F.; Park, Y.N.; Deng, Y.; Kang, Y.; Yang, J.H.; Chang, J.H.; Kim, D.Y.; Kim, J.A.; et al. Sirt1 negatively regulates FcϵRI-mediated mast cell activation through AMPK- and PTP1B-dependent processes. Sci. Rep. 2017, 7, 6444. [Google Scholar] [CrossRef]
- Ito, Y.; Hsu, M.-F.; Bettaieb, A.; Koike, S.; Mello, A.; Calvo-Rubio, M.; Villalba, J.M.; Haj, F.G. Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metabolism 2017, 76, 56–69. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, F.; He, Y.; Zhang, Q.; Zhang, Y.; Zhou, G.; Yang, H.; Zhou, P. A novel PTP1B inhibitor extracted from: Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 2018, 9, 397–406. [Google Scholar] [CrossRef]
- Luo, J.; Hou, Y.; Xie, M.; Ma, W.; Shi, D.; Jiang, B. CYC31, A natural bromophenol PTP1B inhibitor, activates insulin signaling and improves long chain-fatty acid oxidation in C2C12 myotubes jiao. Mar. Drugs 2020, 18, 267. [Google Scholar] [CrossRef]
- Wolfson, B. Adipocyte activation of cancer stem cell signaling in breast cancer. World J. Biol. Chem. 2015, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, X.; Zhao, Q.; Li, Z.; Fu, F.; Zhang, H.; Zheng, M.; Zhang, S. Molecular mechanism of stem cell differentiation into adipocytes and adipocyte differentiation of malignant tumor. Stem Cells Int. 2020, 2020, 8892300. [Google Scholar] [CrossRef]
- Goto, H.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Kono, S.; Takao, S.; Mukohara, T.; Minami, H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 2019, 38, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Gruzewska, K.; Michno, A.; Pawelczyk, T.; Bielarczyk, H. Essentiality and toxicity of vanadium supplements in health and pathology. J. Physiol. Pharmacol. 2014, 65, 603–611. [Google Scholar] [PubMed]
- Zhang, S.; Yan, L.; Kim, S.M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of β-catenin and LKB1/AMPK signaling pathway. PLoS ONE 2020, 15, e0239547. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, Y.; Wang, N.; Wang, Z.; Zhao, P.; Yang, X. Is the hypoglycemic action of vanadium compounds related to the suppression of feeding? Biol. Trace Elem. Res. 2014, 157, 242–248. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Gao, L.; Li, C.; Huan, Y.; Lei, L.; Cao, H.; Li, L.; Gao, A.; Liu, S.; et al. Combination of bis (α-furancarboxylato) oxovanadium (IV) and metformin improves hepatic steatosis through down-regulating inflammatory pathways in high-fat diet-induced obese C57BL/6J mice. Basic Clin. Pharmacol. Toxicol. 2021, 128, 747–757. [Google Scholar] [CrossRef]
- Silva-Nolasco, A.M.; Camacho, L.; Saavedra-Díaz, R.O.; Hernández-Abreu, O.; León, I.E.; Sánchez-Lombardo, I. Kinetic studies of sodium and metforminium decavanadates decomposition and in vitro cytotoxicity and insulin-like activity. Inorganics 2020, 8, 67. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, F.; Zhang, F.; Liu, P.; Xu, T.; Ding, W. Vanadium(IV)-chlorodipicolinate alleviates hepatic lipid accumulation by inducing autophagy via the LKB1/AMPK signaling pathway in vitro and in vivo. J. Inorg. Biochem. 2018, 183, 66–76. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Liu, F.; Zhang, F.; Ding, W. Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J. Inorg. Biochem. 2016, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Chang, H.W. Natural vanadium-containing Jeju ground water stimulates glucose uptake through the activation of AMP-activated protein kinase in L6 myotubes. Mol. Cell. Biochem. 2012, 360, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Park, S.H.; Choi, G.H.; Park, I.J.; Lee, J.H.; Lee, O.H.; Kim, J.H.; Seo, Y.H.; Cho, J.H. Antidiabetic effect of an extract of nutricultured Brassica napus containing vanadium from a Jeju water concentrate. Food Sci. Biotechnol. 2019, 28, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, M.; Zhao, P.; Yang, X. Vanadyl acetylacetonate upregulates PPARγ and adiponectin expression in differentiated rat adipocytes. J. Biol. Inorg. Chem. 2013, 18, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Dong, Y.; Wang, J.; Yang, X. Vanadyl acetylacetonate attenuates Aβ pathogenesis in APP/PS1 transgenic mice depending on the intervention stage. New J. Chem. 2019, 43, 17588–17594. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, X. Vanadium compounds modulate PPARγ activity primarily by increasing PPARγ protein levels in mouse insulinoma NIT-1 cells. Metallomics 2013, 5, 836–843. [Google Scholar] [CrossRef]
- Cheng, H.S.; Yip, Y.S.; Lim, E.K.Y.; Wahli, W.; Tan, N.S. PPARs and tumor microenvironment: The emerging roles of the metabolic master regulators in tumor stromal-epithelial crosstalk and carcinogenesis. Cancers 2021, 13, 2153. [Google Scholar] [CrossRef]
- Kaur, S.; Nag, A.; Gangenahalli, G.; Sharma, K. Peroxisome proliferator activated receptor gamma sensitizes non-small cell lung carcinoma to gamma irradiation induced apoptosis. Front. Genet. 2019, 10, 554. [Google Scholar] [CrossRef] [Green Version]
- Im, C.-N. Targeting glioblastoma stem cells (GSCs) with peroxisome proliferator-activated receptor gamma (PPARγ) ligands. IUBMB Life 2016, 68, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Lanlan, L.; Yang, Z.; Xu, Y.; Li, J.; Xu, D.; Zhang, L.; Sun, J.; Xia, S.; Zou, F.; Liu, Y. Inhibition of oxidative stress-elicited AKT activation facilitates PPARcAgonist-mediated inhibition of stemcell character and tumor growth of liver cancer cells. PLoS ONE 2013, 8, e73038. [Google Scholar]
- Augimeri, G.; Giordano, C.; Gelsomino, L.; Plastina, P.; Barone, I.; Catalano, S.; Andò, S.; Bonofiglio, D. The role of PPARγ ligands in breast cancer: From basic research to clinical studies. Cancers 2020, 12, 2623. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, S.; Momenzadeh, S.; Seyed Forootan, F.; Ghaedi, K.; Nasr Esfahani, M.H. The influence of peroxisome proliferator-activated receptor γ (PPARγ) ligands on cancer cell tumorigenicity. Gene 2018, 649, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Amante, C.; de Sousa-Coelho, A.L.; Aureliano, M. Vanadium and melanoma: A systematic review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Noriega, L.; Castro, M.E.; Perez-Aguilar, J.M.; Caballero, N.A.; Sanchez-Gaytan, B.L.; González-Vergara, E.; Melendez, F.J. Oxidovanadium(V) complexes as promising anticancer photosensitizers. J. Inorg. Biochem. 2020, 203, 110862. [Google Scholar] [CrossRef]
- Molinuevo, M.S.; Cortizo, A.M.; Etcheverry, S.B. Vanadium(IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells. Cancer Chemother. Pharmacol. 2008, 61, 767–773. [Google Scholar] [CrossRef]
- Shi, J.; Wang, D.M.; Wang, C.M.; Hu, Y.; Liu, A.H.; Zhang, Y.L.; Sun, B.; Song, J.G. Insulin receptor substrate-1 suppresses transforming growth factor-β1–mediated epithelial-mesenchymal transition. Cancer Res. 2009, 69, 7180–7187. [Google Scholar] [CrossRef] [Green Version]
- Petanidis, S.; Kioseoglou, E.; Domvri, K.; Zarogoulidis, P.; Carthy, J.M.; Anestakis, D.; Moustakas, A.; Salifoglou, A. In vitro and ex vivo vanadium antitumor activity in (TGF-β)-inducedEMT. Synergistic activity with carboplatin and correlation with tumormetastasis in cancer patients. Int. J. Biochem. Cell Biol. 2016, 74, 121–134. [Google Scholar] [CrossRef]
- Smith, D.M.; Pickering, R.M.; Lewith, G.T. A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. QJM 2008, 101, 351–358. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]







| Drug | Cell Lines | Activated AMPK-Mediated Action | Reference |
|---|---|---|---|
| FND-4b | MCF-7, T-47D, MDA-MB-231, HCC-1143, and HCC-1806 (CSCs) * | Downregulation of ACC, S6, and cyclin D1 activity | [56] |
| Metformin | EC109 and EC9706 | Cell cycle arrest in G0/G1 phase | [65] |
| Simvastatin | HepG2 and Hep3B | G0/G1 arrest by upregulating p21 | [66] |
| Marine sponge-derived smenospongine | MCF7, HBL100, and 16HBE (CSCs) * | Cell cycle arrest and downregulation of Nanog, Bmi1, and Sox2 | [30] |
| Phenformin | MCF7, ZR-75-1, MDA-MB-231, and SUM1315 | Downregulation of cyclin D1, cell cycle arrest at G1 phase, and downregulation of pERK in ER+ cells (MCF7 and ZR-75-1) only | [68] |
| Epigallocatechin gallate and analogues | MDA-MB-231 (CSCs) * Hep G2 and Hep 3B HCT116 and HT-29 | Cell cycle arrest and downregulation of mTOR Downregulation of mTOR and lipogenesis Inhibition of lipogenesis and energy metabolism | [75,78,84] |
| Metformin | MCF-7 and MDA-MB-231 cells MIA PaCa-2 (CSCs) * | Downregulation of cyclin D1, cell cycle arrest at G1 phase, and suppression of mTOR | [87] |
| Metformin | RPMI8226 and U266 | Induction of autophagy and G0/G1 cell cycle arrest and suppression of mTORC1 and mTORC2 | [85] |
| Salinomycin | RB 383, WERI-Rb-1 and RB116 (CSCs) * | Inhibition of mitochondrial respiration and mTOR | [88] |
| AICAR | Glioblastoma, in vivo | Inhibition of lipogenesis and mTOR | [74] |
| MT 63-78 | LNCaP, CL1, PC3, DU145, and HeLa | Inhibition of lipogenesis and mTOR | [77] |
| γ–Tocotrienol | MCF-7 and MDA-MB-231 | Warburg effect | [104] |
| Baicalein | PC-3, DU145, and MDA-MB-231 | Inhibition of mTOR and autophagy | [63] |
| Cyclovirobuxine D | MCF7 | AMPK autophagy | [64] |
| Cucurbitacin E | HeLa, MCF7, and DU145 | AMPK, autophagy, and reduced mTORC1 | [105] |
| Compound | Cell Lines | Action | Reference |
|---|---|---|---|
| Oleuropein | MCF7 | Cytotoxicity | [134] |
| Curcumin and derivatives | MCF-7 and MDA-MB-231 | ROS generation, cytotoxicity | [138] |
| Jamunones | MCF-7 and MDA-MB-231, TNBC | Downregulation of (PI3K)/Akt pathway-mediated apoptosis; G0/G1 phase arrest | [139] |
| Green tea catechins (epigallocatechin and epigallocatechin gallate) | MCF-7 | Cytotoxicity | [140] |
| Alpha-lipoic acid (ALA) and its reduced form of dihydrolipoic acid (DHLA) | MCF7 | PTP and SHP2 inhibition and cytotoxicity | [141] |
| Flavonoids from Orthosiphon stamineus Benth. | MCF7, MCF7/TAMR, and MDA-MB-231 | Cytotoxicity | [142] |
| Docosahexaenoic acid | MCF7 | Cytotoxicity | [143] |
| Oleanane triterpenes from Camellia japonica | MCF7, MCF7/ADR, and MDA-MB-231 | Cytotoxicity | [144] |
| Curcumin and cinnamaldehyde | MCF 7 | Cytotoxicity | [145] |
| Isoflavonoids from Erythrina addisoniae | MCF7, MCF7/ADR, and MDA-MB-231 | Cytotoxicity | [146] |
| Pterocarpan derivatives from Erythrina abyssinica | MCF7, MCF7/TAMR, MCF7/ADR, and MDA-MB-231 | Cytotoxicity | [147] |
| Aurintricarboxylic acid (Fe chelator) | MCF7 | Inhibition of SHP2 phosphatases and cytotoxicity | [148] |
| Compounds | Action | Reference |
|---|---|---|
| BFOV (BFOV + metformin) | Activation of AMPK and reduced hepatic steatosis | [161] |
| (VO(acac)2) | Activation of AMPK, p38, and PPARγ, stimulation of adiponectin | [167,168] |
| Vanadium protein complex | Activation of AMPK and LKB1 and decreased adipogenesis | [159] |
| (VO(dipic-Cl)(H2O)2) | Activation of AMPK/LKB1, autophagy and reduced lipid accumulation and adipogenesis | [163,164] |
| BSOV | Activation of AMPK and PPARγ Insulin mimetic action | [160] |
| Vanadium-containing Jeju groundwater | Activation of AMPK and reduced adipogenesis | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uprety, B.; Abrahamse, H. Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights. Cells 2022, 11, 576. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11030576
Uprety B, Abrahamse H. Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights. Cells. 2022; 11(3):576. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11030576
Chicago/Turabian StyleUprety, Bhawna, and Heidi Abrahamse. 2022. "Targeting Breast Cancer and Their Stem Cell Population through AMPK Activation: Novel Insights" Cells 11, no. 3: 576. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11030576