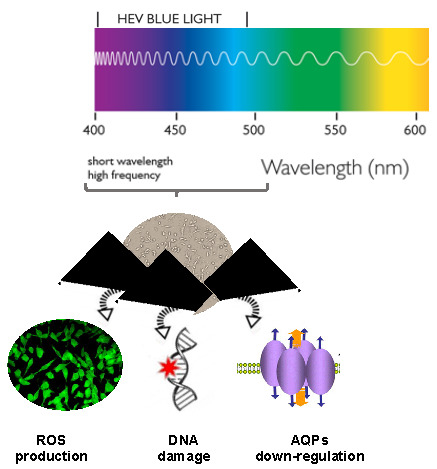
Blue Light Induces Down-Regulation of Aquaporin 1, 3, and 9 in Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatment
2.3. Cell Viability
2.4. Determination of ROS
2.5. Determination of 8-Hydroxy-2’-Deoxyguanosine (8-OHdG)
2.6. RT-PCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. Production of ROS
3.3. Determination of Collagen Type I and DNA Damage
3.4. Determination of AQPs Expression Profile
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mamalis, A.; Koo, E.; Jagdeo, J. Resveratrol prevents reactive oxygen species–induced effects of light-emitting diode–generated blue light in human skin fibroblasts. Dermatol. Surg. 2016, 42, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ayaka, Y.; Fumihiko, Y.; Tetsuya, M.; Yojiro, M.; Kazuyoshi, H.; Chihiro, M.; Satoko, W.T.; Shunsuke, T.; Osamu, T.; Masaichi, C.L. Reactive oxygen species production in mitochondria of human gingival fibroblast induced by blue light irradiation. J. Photochem. Photobiol. B Biol. 2013, 129, 1–5. [Google Scholar]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef] [PubMed]
- Opländer, C.; Hidding, S.; Frauke, B.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.A.; Kang, E.S.; Lee, H.; Hwang, J.S.; Yoo, T.; Paek, K.S.; Park, C.; Kim, J.H.; Lim, D.S.; Seo, H.G. PPAR-delta inhibits UVB-induced secretion of MMP-1 through MKP-7-mediated suppression of JNK signaling. J. Investig. Dermatol. 2013, 133, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Menon, G.K. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991, 24, 1–26. [Google Scholar] [PubMed]
- Brandner, J.M. Pores in the epidermis: Aquaporins and tight junctions. Int. J. Cosmet. Sci. 2007, 29, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. Aquaporin superfamily with unusual NPA boxes: S-aquaporins (superfamily, sip-like and subcellular-aquaporins). Cell. Mol. Biol. 2006, 52, 20–27. [Google Scholar] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell 2005, 97, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Cardile, V. Human mesenchymal stem cells from adipose tissue differentiated into neuronal or glial phenotype express different aquaporins. Mol. Neurobiol. 2017, 54, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.C.E.; Avola, R.; Pannuzzo, G.; Cardile, V. Aquaporin1 and 3 modification as a result of chondrogenic differentiation of human mesenchymal stem cell. J. Cell. Physiol. 2018, 233, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.; Pannuzzo, G.; Alvares, E.; Cardile, V. Krabbe’s leukodystrophy: Approaches and models in vitro. J. Neurosci. Res. 2016, 94, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Albouchi, F.; Cardile, V. New insights on Parkinson’s disease from differentiation of SH-SY5Y into dopaminergic neurons: An involvement of aquaporin4 and 9. Mol. Cell. Neurosci. 2018, 88, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.; Chau, E.; Fillmore, P.; Matthews, J.; Price, L.A.; Sidhaye, V.; Milner, S.M. Epidermal aquaporin-3 is increased in the cutaneous burn wound. Burns 2015, 41, 843–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boury-Jamot, M.; Sougrat, R.; Tailhardat, M.; Le Varlet, B.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim. Biophys. Acta 2006, 1758, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, M.; Daraspe, J.; Daraspe, J.; Bonté, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J. Skin Aquaporins: Function in Hydration, Wound Healing, and Skin Epidermis Homeostasis. Handb. Exp. Pharmacol. 2009, 190, 205–217. [Google Scholar]
- Graziano, A.C.; Parenti, R.; Avola, R.; Cardile, V. Krabbe disease: Involvement of connexin43 in the apoptotic effects of sphingolipid psychosine on mouse oligodendrocyte precursors. Apoptosis 2016, 21, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.C.; Cardile, V.; Crascì, L.; Caggia, S.; Dugo, P.; Bonina, F.; Panico, A. Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-gamma and histamine exposed human keratinocytes. Life Sci. 2012, 90, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.C.E.; Pannuzzo, G.; Salemi, E.; Santagati, A.; Avola, R.; Longo, E.; Cardile, V. Synthesis, characterization, molecular modeling, and biological evaluation of thieno-pyrimidinone methanesulphonamide thio-derivatives as nonsteroidal anti-inflammatory agents. Clin. Exp. Pharmacol. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Avola, R.; Graziano, A.C.E.; Piovano, M.; Russo, A. Cytotoxicity of demalonyl thyrsiflorin A, a semisynthetic labdane-derived diterpenoid, to melanoma cells. Toxicol. In Vitro 2018, 47, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Carney, J.M. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999, 9, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.C.; Silva, K.C.; Biswas, S.K.; Martins, N.; De Faria, J.B.; De Faria, J.M. Arterial hypertension exacerbates oxidative stress in early diabetic retinopathy. Free Radic. Res. 2007, 41, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Celis, J.E.; Madsen, P. Increased nuclear cyclin/PCNA antigen staining of non-S phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986, 209, 277–283. [Google Scholar] [CrossRef]
- Rascalou, A.; Lamartine, J.; Poydenot, P.; Demarne, F.; Bechetoille, N. Mitochondrial damage and cytoskeleton reorganization in human dermal fibroblasts exposed to artificial visible light similar to screen-emitted light. J. Dermatol. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti-Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Mera, S.L.; Lovell, C.R.; Jones, R.R.; Davies, J.D. Elastic fibres in normal and sun-damaged skin: An immunohistochemical study. Br. J. Dermatol. 1987, 117, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kumud, M.; Sanju, N. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar]
- Monestier, S.; Gaudy, C.; Gouvernet, J.; Richard, M.A.; Grob, J.J. Multiple senile lentigos of the face, a skin-ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned Caucasians: A case-control study. Br. J. Dermatol. 2006, 154, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Akiba, S.; Shinkura, R.; Miyamoto, K.; Hillebrand, G.; Yamaguchi, N.; Ichihashi, M. Influence of chronic UV exposure and lifestyle on facial skin photo-aging—Results from a pilot study. J. Epidemiol. 1999, 9, 136–142. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radical Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.; Russell, E.; Latimer, J. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013, 169, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Can, C.; Shu, W.; Qin, J.; Ashley, A.; Shan, L.; Gang, H.; Zhigang, B.; Kouttab, N.; Chu, W.; Wan, Y. All-Trans Retinoic Acid Attenuates Ultraviolet Radiation-Induced Down-Regulation of Aquaporin-3 and Water Permeability in Human Keratinocytes. J. Cell. Physiol. 2008, 215, 506–516. [Google Scholar]
- Verkman, A.S. Aquaporin water channels and endothelial cell function. J. Anat. 2002, 200, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Verkman, A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7360–7365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.M.; Verkman, A.S. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kwon, T.E.; Kim, S.D.; Park, S.B.; Kwon, O.S.; Kim, K.H.; Park, K.C. Changes of transepidermal water loss (TEWL) in psoriatic plaques during D-PUVA therapy. Ann. Dermatol. 2001, 13, 7–11. [Google Scholar] [CrossRef]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentini, D.; Zambonin, L.; Vieceli Dalla Sega, F.; Hrelia, S. Polyphenols as modulators of aquaporin family in health and disease. Oxidative Med. Cell. Longevity 2015. [Google Scholar] [CrossRef] [PubMed]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. Glycerol and urea can be used to increase skin permeability in reduced hydration conditions. Eur. J. Pharm. Sci. 2013, 50, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Kohei, Y.; Kusaka-Kikushima, A.; Nakahigashi, K.; Hagiwara, H.; Miyach, I.Y. Analysis of aquaporin 9 expression in human epidermis and cultured keratinocytes. FEBS Open Bio 2014, 4, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim. Biophys. Acta 2001, 1522, 82–88. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, S.A. Aquaporins and cell migration. Pflugers Arch. 2000, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins: Translating bench research to human disease. J. Exp. Biol. 2009, 212, 1707–1715. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Cardile, V. Blue Light Induces Down-Regulation of Aquaporin 1, 3, and 9 in Human Keratinocytes. Cells 2018, 7, 197. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7110197
Avola R, Graziano ACE, Pannuzzo G, Cardile V. Blue Light Induces Down-Regulation of Aquaporin 1, 3, and 9 in Human Keratinocytes. Cells. 2018; 7(11):197. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7110197
Chicago/Turabian StyleAvola, Rosanna, Adriana Carol Eleonora Graziano, Giovanna Pannuzzo, and Venera Cardile. 2018. "Blue Light Induces Down-Regulation of Aquaporin 1, 3, and 9 in Human Keratinocytes" Cells 7, no. 11: 197. https://0-doi-org.brum.beds.ac.uk/10.3390/cells7110197