Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
Abstract
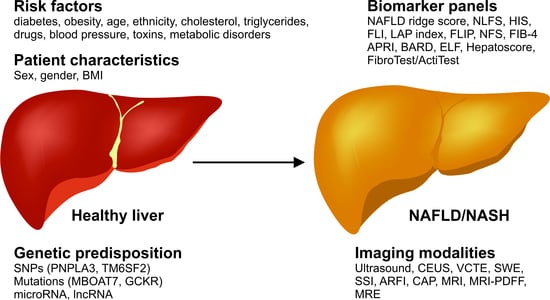
:1. Introduction
2. Blood and Serum Tests
2.1. Steatosis
2.2. Steatohepatitis
2.3. Fibrosis
3. Imaging
3.1. Ultrasound
3.2. Ultrasound-Based Elastography Techniques
3.3. Controlled Attenuation Parameter
3.4. Magnetic Resonance Imaging in NASH and NAFLD
3.5. Magnetic Resonance Elastography
4. Genetic Tests
5. Screening for NAFLD and NASH
6. Other Factors in NAFLD and NASH
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Byron, D.; Minuk, G.Y. Clinical hepatology: Profile of an urban, hospital-based practic. Hepatology 1996, 24, 813–815. [Google Scholar] [CrossRef] [PubMed]
- James, O.F.; Day, C.P. Non-alcoholic steatohepatitis (NASH): A disease of emerging identity and importance. J. Hepatol. 1998, 29, 495–501. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Wong, R.J.; Gish, R.G. Diagnostic and treatment implications of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Gastroenterol. Hepatol. 2019, 15, 83–89. [Google Scholar]
- Angulo, P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007, 65, 57–63. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Genetics of nonalcoholic fatty liver disease: From pathogenesis to therapeutics. Semin. Liver Dis. 2019, 39, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Le Guillou, D.; Begriche, K.; Fromenty, B. Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Charlotte, F.; Perazzo, H.; Ngo, Y.; Deckmyn, O.; Pais, R.; Mathurin, P.; Ratziu, V. FLIP consortium, the FibroFrance-CPAM group; FibroFrance-Obese group. Impact of steatosis and inflammation definitions on the performance of NASH tests. Eur. J. Gastroenterol. Hepatol. 2018, 30, 384–391. [Google Scholar] [CrossRef]
- Lykke Eriksen, P.; Sørensen, M.; Grønbæk, H.; Hamilton-Dutoit, S.; Vilstrup, H.; Thomsen, K.L. Non-alcoholic fatty liver disease causes dissociated changes in metabolic liver functions. Clin. Res. Hepatol Gastroenterol. 2019, 13. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Tacke, F. The role of radiologic modalities in diagnosing nonalcoholic steatohepatitis (NASH) and fibrosis. Curr. Hepatol. Rep. 2018, 17, 324–335. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Schon, H.-T.; Weiskirchen, R. Exercise-induced release of pharmacologically active substances and their relevance for therapy of hepatic injury. Front. Pharmacol. 2016, 7, 1101. [Google Scholar] [CrossRef]
- Jeznach-Steinhagen, A.; Ostrowska, J.; Czerwonogrodzka-Senczyna, A.; Boniecka, I.; Shahnazaryan, U.; Kuryłowicz, A. Dietary and pharmacological treatment of nonalcoholic fatty liver disease. Medicina 2019, 55, 166. [Google Scholar] [CrossRef]
- Diwan, T.S.; Rice, T.C.; Heimbach, J.K.; Schauer, D.P. Liver transplantation and bariatric surgery: Timing and outcomes. Liver Transplant. 2018, 24, 1280–1287. [Google Scholar] [CrossRef]
- Conway, B.N.; Han, X.; Munro, H.M.; Gross, A.L.; Shu, X.-O.; Hargreaves, M.K.; Zheng, W.; Powers, A.C.; Blot, W.J. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PLoS ONE 2018, 13, e0190993. [Google Scholar] [CrossRef]
- Hirode, G.; Vittinghoff, E.; Wong, R.J. Increasing clinical and economic burden of nonalcoholic fatty liver disease among hospitalized adults in the United States. J. Clin. Gastroenterol. 2019, 10, 1097. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J. Hepatol 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Cholankeril, G.; Wong, R.J.; Hu, M.; Perumpail, R.B.; Yoo, E.R.; Puri, P.; Younossi, Z.M.; Harrison, S.A.; Ahmed, A. Liver transplantation for nonalcoholic steatohepatitis in the US: Temporal trends and outcomes. Dig. Dis. Sci. 2017, 62, 2915–2922. [Google Scholar] [CrossRef]
- Bril, F.; Kalavalapalli, S.; Clark, V.C.; Lomonaco, R.; Soldevila-Pico, C.; Liu, I.C.; Orsak, B.; Tio, F.; Cusi, K. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs. without type 2 diabetes. Clin. Gastroenterol. Hepatol. 2018, 16, 558–560. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Sharma, B.C.; Kumar, A.; Garg, V.; Reddy, R.S.; Sakhuja, P.; Sarin, S.K. A Randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis. J. Clin. Exp. Hepatol. 2012, 2, 333–337. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Chitturi, S.; Wong, G.L.-H.; Yu, J.; Chan, H.L.-Y.; Farrell, G.C.; Chitturi, S. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol. 2016, 1, 56–67. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Townsend, S.; Newsome, P.N. Non-alcoholic fatty liver disease in 2016. Br. Med Bull. 2016, 119, 143–156. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.-S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Lifestyle modification as the primary treatment of NASH. Clin. Liver Dis. 2009, 13, 649–665. [Google Scholar] [CrossRef]
- Tacke, F.; Weiskirchen, R. An update on the recent advances in antifibrotic therapy. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary causes of nonalcoholic fatty liver disease. Therap. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef]
- Paschetta, E.; Belci, P.; Alisi, A.; Liccardo, D.; Cutrera, R.; Musso, G.; Nobili, V. OSAS-related inflammatory mechanisms of liver injury in nonalcoholic fatty liver disease. Mediat. Inflamm. 2015, 2015, 815721. [Google Scholar] [CrossRef]
- Macut, D.; Božić-Antić, I.; Bjekić-Macut, J.; Tziomalos, K. Management of endocrine disease: Polycystic ovary syndrome and nonalcoholic fatty liver disease. Eur. J. Endocrinol. 2017, 177, R145–R158. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Younes, R.; Bugianesi, E. NASH in lean individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef]
- Koch, L.K.; Yeh, M.M. Nonalcoholic fatty liver disease (NAFLD): Diagnosis, pitfalls, and staging. Ann. Diagn. Pathol. 2018, 37, 83–90. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- McGill, D.B.; Rakela, J.; Zinsmeister, A.R.; Ott, B.J. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990, 99, 1396–1400. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; Bhanpuri, N.H.; McKenzie, A.L.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S.; et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: An open-label, non-randomised controlled study. BMJ Open 2019, 9, e023597. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Ma, A.J.; Wong, V.W.-S.; Tse, Y.-K.; Chan, H.L.-Y.; Yuen, P.-C.; Wong, G.L.-H.; Yip, T.C.; Wong, V.W.; Tse, Y.; et al. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment. Pharmacol. Ther. 2017, 46, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Yip, T.C.-F.; Wong, V.W.-S.; Yip, T.C.; Wong, V.W. How to identify patients with advanced liver disease in the community? Hepatology 2017, 66, 7–9. [Google Scholar] [CrossRef]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Calori, G.; Lattuada, G.; Ragogna, F.; Garancini, M.P.; Crosignani, P.; Villa, M.; Bosi, E.; Ruotolo, G.; Piemonti, L.; Perseghin, G. Fatty liver index and mortality: The cremona study in the 15th year of follow-up. Hepatology 2011, 54, 145–152. [Google Scholar] [CrossRef]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef]
- Bedossa, P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Canbay, A.; Kälsch, J.; Neumann, U.; Rau, M.; Hohenester, S.; Baba, H.A.; Rust, C.; Geier, A.; Heider, D.; Sowa, J.-P. Non-invasive assessment of NAFLD as systemic disease—A machine learning perspective. PLoS ONE 2019, 14, e0214436. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- McPherson, S.; Henderson, E.; Stewart, S.F.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Kruger, F.C.; Daniels, C.R.; Kidd, M.; Swart, G.; Brundyn, K.; Van Rensburg, C.; Kotze, M. APRI: A simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S. Afr. Med. J. 2011, 101, 477–480. [Google Scholar]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; A Neuschwander-Tetri, B. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef]
- Rosenberg, W.M.; Voelker, M.; Thiel, R.; Becka, M.; Burt, A.; Schuppan, D.; Hubscher, S.; Roskams, T.; Pinzani, M.; Arthur, M.J. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004, 127, 1704–1713. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.A.; Bulsara, M.; Rossi, E.; DeBoer, B.; Speers, D.; George, J.; Kench, J.; Farrell, G.; McCaughan, G.W.; Jeffrey, G.P. Hepascore: An accurate validated predictor of liver fibrosis in chronic hepatitis C Infection. Clin. Chem. 2005, 51, 1867–1873. [Google Scholar] [CrossRef]
- Poynard, T.; Imbert-Bismut, F.; Munteanu, M.; Ratziu, V. FibroTest-FibroSURE™: Towards a universal biomarker of liver fibrosis? Expert Rev. Mol. Diagn. 2005, 5, 15–21. [Google Scholar] [CrossRef]
- Cales, P.; Oberti, F.; Michalak, S.; Rousselet, M.-C.; Konaté, A.; Gallois, Y.; Ternisien, C.; Chevailler, A.; Lunel, F.; Hubert-Fouchard, I.; et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005, 42, 1373–1381. [Google Scholar] [CrossRef]
- Boursier, J.; Guillaume, M.; Leroy, V.; Irlès, M.; Roux, M.; Lannes, A.; Foucher, J.; Zuberbuhler, F.; Delabaudière, C.; Barthelon, J.; et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J. Hepatol. 2019, 71, 389–396. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Zubiaga, L. Validation of biochemical scores for liver steatosis before and 1 year after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2019. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Lam, K.S.; Wong, I.C.; Cheung, B.M. Non-invasive score identifies ultrasonography-diagnosed non-alcoholic fatty liver disease and predicts mortality in the USA. BMC Med. 2014, 12, 154. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Wieckowska, A.; Lopez, A.R.; Liu, Y.-C.; Zein, N.N.; McCullough, A.J. Cytokeratin-18 fragment levels as noninvasive biomarker for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology 2009, 50, 1072–1078. [Google Scholar] [CrossRef]
- Eguchi, A.; Wree, A.; Feldstein, A.E. Biomarkers of liver cell death. J. Hepatol. 2014, 60, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- Church, R.J.; Watkins, P.B. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int. 2017, 37, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Chang, M.; Bhaskar, K.; Nasser, I.; Curry, M.; Schuppan, D.; Byrnes, V.; Afdhal, N. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2009, 24, 564–568. [Google Scholar] [CrossRef]
- Kwok, R.; Tse, Y.K.; Wong, G.L.; Ha, Y.; Lee, A.U.; Ngu, M.C.; Chan, H.L.; Wong, V.W. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 2014, 39, 254–269. [Google Scholar] [CrossRef]
- He, L.; Deng, L.; Zhang, Q.; Guo, J.; Zhou, J.; Song, W.; Yuan, F. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: A systematic review and meta-analysis. BioMed Res. Int. 2017, 2017, 9729107. [Google Scholar] [CrossRef]
- Tamimi, T.I.A.-R.; Elgouhari, H.M.; Alkhouri, N.; Yerian, L.M.; Berk, M.P.; Lopez, R.; Schauer, P.R.; Zein, N.N.; Feldstein, A.E. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J. Hepatol. 2011, 54, 1224–1229. [Google Scholar] [CrossRef]
- Jarrar, M.H.; Baranova, A.; Collantes, R.; Ranard, B.; Stepanova, M.; Bennett, C.; Fang, Y.; Elariny, H.; Goodman, Z.; Chandhoke, V.; et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2008, 27, 412–421. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Fang, Q.; Zhang, J.; Zhang, M.; Zhang, L.; Wu, L.; Hou, X.; Lu, J.; Bao, Y.; et al. Complementary Role of fibroblast growth factor 21 and cytokeratin 18 in monitoring the different stages of nonalcoholic fatty liver disease. Sci. Rep. 2017, 7, 5095. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Gao, F.; Fan, J.; Zhou, J.; Wang, X.; Zhang, H.; Pan, X.; Bao, Y.; Xiang, K.; et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J. Hepatol. 2010, 53, 934–940. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Eren, F.; Yonal, O.; Kurt, R.; Aktas, B.; Celikel, C.A.; Özdoğan, O.; Imeryuz, N.; Kalayci, C.; Avsar, E.; et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2010, 40, 887–892. [Google Scholar] [CrossRef]
- Gariani, K.; Drifte, G.; Dunn-Siegrist, I.; Pugin, J.; Jornayvaz, F.R. Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocr. Connect. 2013, 2, 146–153. [Google Scholar] [CrossRef]
- Yan, H.; Xia, M.; Chang, X.; Xu, Q.; Bian, H.; Zeng, M.; Rao, S.-X.; Yao, X.; Tu, Y.; Jia, W.; et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: A cross-sectional study. PLoS ONE 2011, 6, e24895. [Google Scholar] [CrossRef]
- Lau, H.H.; Ng, N.H.J.; Loo, L.S.W.; Jasmen, J.B.; Teo, A.K.K. The molecular functions of hepatocyte nuclear factors – In and beyond the liver. J. Hepatol. 2018, 68, 1033–1048. [Google Scholar] [CrossRef]
- Moya, M.; Benet, M.; Guzmán, C.; Tolosa, L.; García-Monzón, C.; Pareja, E.; Castell, J.V.; Jover, R. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS ONE 2012, 7, e30014. [Google Scholar] [CrossRef]
- Ramadori, P.; Drescher, H.; Erschfeld, S.; Schumacher, F.; Berger, C.; Fragoulis, A.; Schenkel, J.; Kensler, T.W.; Wruck, C.J.; Trautwein, C.; et al. Hepatocyte-specific Keap1 deletion reduces liver steatosis but not inflammation during non-alcoholic steatohepatitis development. Free. Radic. Boil. Med. 2016, 91, 114–126. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Lopez, R.; Tamimi, T.A.-R.; Yerian, L.; Chung, Y.-M.; Berk, M.; Zhang, R.; McIntyre, T.M.; Hazen, S.L. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 2010, 51, 3046–3054. [Google Scholar] [CrossRef] [Green Version]
- Hagström, H.; Stål, P.; Hultcrantz, R.; Brismar, K.; Ansurudeen, I. IGFBP-1 and IGF-I as markers for advanced fibrosis in NAFLD – a pilot study. Scand. J. Gastroenterol. 2017, 52, 1427–1434. [Google Scholar] [CrossRef]
- Regué, L.; Minichiello, L.; Avruch, J.; Dai, N. Liver-specific deletion of IGF2 mRNA binding protein-2/IMP2 reduces hepatic fatty acid oxidation and increases hepatic triglyceride accumulation. J. Boil. Chem. 2019, 294, 11944–11951. [Google Scholar] [CrossRef] [Green Version]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E.; Network, N.C.R. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef]
- Maliken, B.D.; Nelson, J.E.; Klintworth, H.M.; Beauchamp, M.; Yeh, M.M.; Kowdley, K.V. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatology 2013, 57, 1806–1813. [Google Scholar] [CrossRef] [Green Version]
- Ajmera, V.; Perito, E.R.; Bass, N.M.; Terrault, N.A.; Yates, K.P.; Gill, R.; Loomba, R.; Diehl, A.M.; Aouizerat, B.E.; Network, N.C.R. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology 2017, 65, 65–77. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Charlotte, F.; Messous, D.; Munteanu, M.; Imbert-Bismut, F.; Massard, J.; Bonyhay, L.; Tahiri, M.; Thabut, D.; et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006, 6, 34. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between non-invasive fibrosis markers and mortality among adults with non-alcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef]
- Cheah, M.C.; McCullough, A.J.; Goh, G.B.-B. Current modalities of fibrosis assessment in non-alcoholic fatty liver disease. J. Clin. Transl. Hepatol. 2017, 5, 261–271. [Google Scholar] [CrossRef]
- Chuah, K.-H.; Yusoff, W.N.I.W.; Sthaneshwar, P.; Mustapha, N.R.N.; Mahadeva, S.; Chan, W.-K. MACK-3 (combination of hoMa, Ast and CK18): A promising novel biomarker for fibrotic non-alcoholic steatohepatitis. Liver Int. 2019, 39, 1315–1324. [Google Scholar] [CrossRef]
- Lydatakis, H.; Hager, I.P.; Kostadelou, E.; Mpousmpoulas, S.; Pappas, S.; Diamantis, I. Non-invasive markers to predict the liver fibrosis in non-alcoholic fatty liver disease. Liver Int. 2006, 26, 864–871. [Google Scholar] [CrossRef]
- Tanwar, S.; Trembling, P.M.; Guha, I.N.; Parkes, J.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; Day, C.P.; Rosenberg, W.M. Validation of terminal peptide of procollagen III for the detection and assessment of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease. Hepatology 2013, 57, 103–111. [Google Scholar] [CrossRef]
- Abdelaziz, R.; Elbasel, M.; Esmat, S.; Essam, K.; Abdelaaty, S. Tissue inhibitors of metalloproteinase-1 and 2 and obesity related non-alcoholic fatty liver disease: Is there a relationship? Digestion 2015, 92, 130–137. [Google Scholar] [CrossRef]
- Santos, V.N.; Leite-Mór, M.M.; Kondo, M.; Martins, J.R.; Nader, H.; Lanzoni, V.P.; Parise, E.R. Serum laminin, type IV collagen and hyaluronan as fibrosis markers in non-alcoholic fatty liver disease. Braz. J. Med. Biol. Res. 2005, 38, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.-S.; Adams, L.A.; De Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Boursier, J.; De Lédinghen, V.; Poynard, T.; Guechot, J.; Carrat, F.; Leroy, V.; Wong, G.L.-H.; Friedrich-Rust, M.; Fraquelli, M.; Plebani, M.; et al. An extension of STARD statements for reporting diagnostic accuracy studies on liver fibrosis tests: The Liver-FibroSTARD standards. J. Hepatol. 2015, 62, 807–815. [Google Scholar] [CrossRef]
- Guechot, J.; Boursier, J.; De Lédinghen, V.; Poynard, T.; Carrat, F.; Leroy, V.; Wong, G.L.-H.; Friedrich-Rust, M.; Fraquelli, M.; Plebani, M.; et al. Liver-FibroSTARD checklist and glossary: Tools for standardized design and reporting of diagnostic accuracy studies of liver fibrosis tests. Clin. Chem. Lab. Med. 2015, 53, 1135–1137. [Google Scholar] [CrossRef]
- Tarantino, G.; Porcu, C.; Arciello, M.; Andreozzi, P.; Balsano, C. Prediction of carotid intima-media thickness in obese patients with low prevalence of comorbidities by serum copper bioavailability. J. Gastroenterol. Hepatol. 2018, 33, 1511–1517. [Google Scholar] [CrossRef]
- Aigner, E.; Strasser, M.; Haufe, H.; Sonnweber, T.; Hohla, F.; Stadlmayr, A.; Solioz, M.; Tilg, H.; Patsch, W.; Weiss, G.; et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2010, 105, 1978–1985. [Google Scholar] [CrossRef]
- Myers, R.P.; Fong, A.; Shaheen, A.A.M. Utilization rates, complications and costs of percutaneous liver biopsy: A population-based study including 4275 biopsies. Liver Int. 2008, 28, 705–712. [Google Scholar] [CrossRef]
- Taylor, K.J.; Gorelick, F.S.; Rosenfield, A.T.; A Riely, C. Ultrasonography of alcoholic liver disease with histological correlation. Radiology 1981, 141, 157–161. [Google Scholar] [CrossRef]
- Mishra, P.; Younossi, Z.M. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am. J. Gastroenterol. 2007, 102, 2716–2717. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Khanal, U.P.; Paudel, B.; Gurung, G.; Hu, Y.S.; Kuo, C.W. Correlational study of nonalcoholic fatty liver disease diagnosed by ultrasonography with lipid profile and body mass index in adult Nepalese population. J. Med. Ultrasound 2019, 27, 19–25. [Google Scholar] [CrossRef]
- Battaglia, V.; Cervelli, R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). Eur. J. Radiol. 2017, 96, 65–73. [Google Scholar] [CrossRef]
- Sandrin, L.; Tanter, M.; Catheline, S.; Fink, M. Shear modulus imaging with 2-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2002, 49, 426–435. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Luckina, E.; Perazzo, H.; Ngo, Y.; Royer, L.; Fedchuk, L.; Sattonnet, F.; Pais, R.; Lebray, P.; et al. Liver fibrosis evaluation using real-time shear wave elastography: Applicability and diagnostic performance using methods without a gold standard. J. Hepatol. 2013, 58, 928–935. [Google Scholar] [CrossRef]
- Afdhal, N.H. Fibroscan (Transient Elastography) for the measurement of liver fibrosis. Gastroenterol. Hepatol. 2012, 8, 605–607. [Google Scholar]
- Palmeri, M.L.; Wang, M.H.; Rouze, N.C.; Abdelmalek, M.F.; Guy, C.D.; Moser, B.; Diehl, A.M.; Nightingale, K.R. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J. Hepatol. 2011, 55, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Karlas, T.; Dietrich, A.; Peter, V.; Wittekind, C.; Lichtinghagen, R.; Garnov, N.; Linder, N.; Schaudinn, A.; Busse, H.; Prettin, C.; et al. Evaluation of transient elastography, acoustic radiation force impulse imaging (ARFI), and enhanced liver function (ELF) score for detection of fibrosis in morbidly obese patients. PLoS ONE 2015, 10, e0141649. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Herrmann, E.; de Ledinghen, V.; Cassinotto, C.; Chu, W.C.; Leung, V.Y.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef]
- Alkhouri, N.; Sedki, E.; Alisi, A.; Lopez, R.; Pinzani, M.; Feldstein, A.E.; Nobili, V. Combined paediatric NAFLD fibrosis index and transient elastography to predict clinically significant fibrosis in children with fatty liver disease. Liver Int. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef]
- Sasso, M.; Beaugrand, M.; De Lédinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Thiele, M.; Jansen, C.; Madsen, B.S.; Görtzen, J.; Strassburg, C.; Trebicka, J.; Krag, A. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS ONE 2017, 12, e0173992. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Non-invasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef]
- Reeder, S.B.; Hu, H.H.; Sirlin, C.B. Proton Density Fat-Fraction: A Standardized MR-Based Biomarker of Tissue Fat Concentration. J. Magn. Reson. Imaging 2012, 36, 1011–1014. [Google Scholar] [CrossRef]
- Wang, X.; Hernando, D.; Reeder, S.B. Sensitivity of chemical shift-encoded fat quantification to calibration of fat MR spectrum. Magn. Reson. Med. 2016, 75, 845–851. [Google Scholar] [CrossRef]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016, 150, 626–637. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Alquiraish, M.; Valasek, M.A.; et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017, 152, S70. [Google Scholar] [CrossRef]
- Runge, J.H.; Smits, L.P.; Verheij, J.; Depla, A.; Kuiken, S.D.; Baak, B.C.; Nederveen, A.J.; Beuers, U.; Stoker, J. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology 2018, 286, 547–556. [Google Scholar] [CrossRef]
- Singh, S.; Venkatesh, S.K.; Wang, Z.; Miller, F.H.; Motosugi, U.; Low, R.N.; Hassanein, T.; Asbach, P.; Godfrey, E.M.; Yin, M.; et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin. Gastroenterol. Hepatol. 2015, 13, 440–451. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef]
- Loomba, R.; Cui, J.; Wolfson, T.; Haufe, W.; Hooker, J.; Szeverenyi, N.; Ang, B.; Bhatt, A.; Wang, K.; Aryafar, H.; et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: A prospective study. Am. J. Gastroenterol. 2016, 111, 986–994. [Google Scholar] [CrossRef]
- Costa-Silva, L.; Ferolla, S.M.; Lima, A.S.; Vidigal, P.V.T.; Ferrari, T.C.D.A. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur. J. Radiol. 2018, 98, 82–89. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Pingitore, P.; Pirazzi, C.; Mancina, R.M.; Motta, B.M.; Indiveri, C.; Pujia, A.; Montalcini, T.; Hedfalk, K.; Romeo, S. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, P.K.; Zhou, Y.; Haridas, P.N.; Dwivedi, O.P.; Hyötyläinen, T.; Ali, A.; Juuti, A.; Leivonen, M.; Tukiainen, T.; Ahonen, L.; et al. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J. Hepatol. 2017, 67, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef]
- Beer, N.L.; Tribble, N.D.; McCulloch, L.J.; Roos, C.; Johnson, P.R.; Orho-Melander, M.; Gloyn, A.L. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum. Mol. Genet. 2009, 18, 4081–4088. [Google Scholar] [CrossRef] [Green Version]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Joshi-Barve, S.; Kirpich, I.; Cave, M.C.; Marsano, L.S.; McClain, C.J. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: Mechanistic similarities and differences. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 356–367. [Google Scholar] [CrossRef]
- Roy, S.; Trautwein, C.; Luedde, T.; Roderburg, C. A General Overview on non-coding RNA-based diagnostic and therapeutic approaches for liver diseases. Front. Pharmacol. 2018, 9, 805. [Google Scholar] [CrossRef]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The role of miRNAs in the pathophysiology of liver diseases and toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Wu, X.; Jiang, L.; Yang, S.; Ding, Z.; Fang, Z.; Hua, H.; Kirby, M.S.; Shou, J. A circulating microRNA signature as noninvasive diagnostic and prognostic biomarkers for nonalcoholic steatohepatitis. BMC Genom. 2018, 19, 188. [Google Scholar] [CrossRef]
- Turchinovich, A.; Baranova, A.; Drapkina, O.; Tonevitsky, A. Cell-free circulating nucleic acids as early biomarkers for NAFLD and NAFLD-associated disorders. Front. Physiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J. Hepatol. 2013, 59, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Meng, F.; Glaser, S.; Alpini, G. Exosomes in liver pathology. J. Hepatol. 2016, 65, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.; Kim, J.; Jung, Y. Liver-derived exosomes and their implications in liver pathobiology. Int. J. Mol. Sci. 2018, 19, 3715. [Google Scholar] [CrossRef]
- Eguchi, A.; Feldstein, A.E. Extracellular vesicles in non-alcoholic and alcoholic fatty liver diseases. Liver Res. 2018, 2, 30–34. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, S.Y.; Ko, E.; Lee, J.-H.; Yi, H.-S.; Yoo, Y.J.; Je, J.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci. Rep. 2017, 7, 3710. [Google Scholar] [CrossRef]
- Korf, H.; Boesch, M.; Meelberghs, L.; Van Der Merwe, S. Macrophages as key players during adipose tissue–liver crosstalk in nonalcoholic fatty liver disease. Semin. Liver Dis. 2019, 39, 291–300. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef]
- Jayakumar, S.; Loomba, R. Review article: Emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Aliment. Pharmacol. Ther. 2019, 50, 144–158. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]




| Panel | Parameters Included | Reference |
|---|---|---|
| NAFLD ridge score | ALT, HDL, cholesterol, triglycerides, HbA1c, leukocyte count, hypertension | [43,44] |
| NAFLD Liver Fat Score (NLFS) | Liver fat content, metabolic syndrome, type 2 diabetes, AST, AST:ALT, fasting insulin level | [45] |
| Hepatic Steatosis Index (HIS) | AST:ALT, BMI, diabetes, sex | [46] |
| Fatty liver index (FLI) | BMI, waist circumference, triglycerides, γ-GT | [47,48] |
| Lipid accumulation product (LAP) index | sex, triglycerides, weight circumference | [49] |
| Fatty Liver Inhibition of Progression (FLIP) algorithm | histological steatosis, disease activity, fibrosis score | [50] |
| CHeK score | age, HbA1c, γ-GT, adiponectin, M30 | [51] |
| NAFLD Fibrosis Score (NFS) | AST:ALT, albumin, platelet count, age, BMI, hyperglycemia (impaired fasting glucose) | [52] |
| Fibrosis-4 Score (FIB-4) | AST, ALT, platelet count, age | [53] |
| AST to Platelet Ratio Index (APRI) | AST:platelet count | [54] |
| BARD Score | BMI, AST:ALT, diabetes | [55] |
| Enhanced Liver Fibrosis panel (ELF) | Age, TIMP-1, PIIINP, HA | [56] |
| Hepascore | bilirubin, γ-GT, HA, α2M, age, gender | [57] |
| FibroTest-FibroSURE/ActiTest | α2M, haptoglobin, γ-GT, total bilirubin, apolipoprotein A1, ALT, age, gender | [58] |
| FibroMeter NAFLD index (FibroMeterVCTE) | platelet count, prothrombin index, ferritin, AST, ALT, body weight, age, liver stiffness determined by vibration controlled transient elastography (VCTE) | [59,60] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Cells 2019, 8, 845. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080845
Drescher HK, Weiskirchen S, Weiskirchen R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Cells. 2019; 8(8):845. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080845
Chicago/Turabian StyleDrescher, Hannah K., Sabine Weiskirchen, and Ralf Weiskirchen. 2019. "Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)" Cells 8, no. 8: 845. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8080845