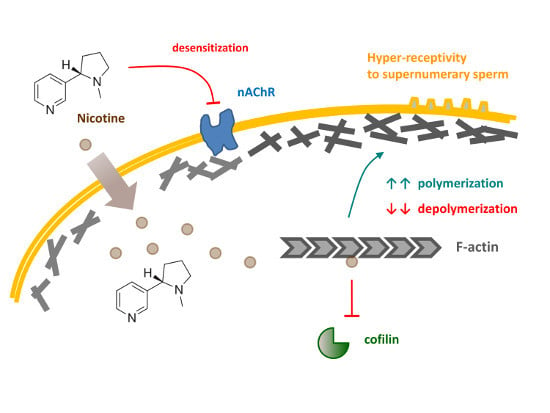
Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamete Preparation and Fertilization
2.2. Reagents and Chemicals
2.3. Microinjection, Confocal Microscopy, and Ca2+ Imaging
2.4. Scanning Electron Microscopy (SEM)
2.5. In Vitro Assay of Actin Polymerization and Depolymerization Kinetics
2.6. Statistical Analysis
3. Results
3.1. Nicotine Induces Polyspermy in a Dose-Dependent Manner
3.2. Nicotine-Induced Polyspermy Is Neither Mimicked by Cholinergic Agonists Nor Inhibited by Antagonists of Nicotinic Acetylcholine Receptors
3.3. Effects of Nicotine Pretreatment on Ca2+ Signaling in the Fertilized Eggs of P. lividus
3.4. Nicotine Hyperpolymerizes Subplasmalemmal Actin Filaments of the Sea Urchin Eggs in a Dose-Dependent Manner
3.5. Nicotine Renders Egg surface Hyper-Receptive to Sperm
3.6. Nicotine Has an Intracellular Target That Is Not Nicotinic AChR
3.7. Nicotine Has a Direct Impact on Actin Dynamics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | cortical flash |
| nAChR | nicotinic acetylcholine receptor |
| FE | fertilization envelope |
References
- Briggs, E.; Wessel, G.M. In the beginning animal fertilization and sea urchin development. Dev. Biol. 2006, 300, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santella, L.; Vasilev, F.; Chun, J.T. Fertilization in echinoderms. Biochem. Biophys. Res. Commun. 2012, 425, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 10th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014; pp. 117–151. [Google Scholar]
- Fankhauser, G. The organization of the amphibian egg during fertilization and cleavage. Ann. N. Y. Acad. Sci. 1948, 49, 684–708. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.J. Fertilization, 2nd ed.; Chapman & Hall: Cambridge, UK, 1997; pp. 65–117. [Google Scholar]
- Snook, R.R.; Hosken, D.J.; Karr, T.L. The biology and evolution of polyspermy: Insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction 2011, 142, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Dale, B.; Monroy, A. How is polyspermy prevented? Gamete Res. 1981, 4, 151–169. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Gould, M. Polyspermy-preventing mechanisms. In Biology of Fertilization: The Fertilization Response of the Egg; Metz, C.B., Monroy, A., Eds.; Academic Press: Orlando, FL, USA, 1985; pp. 223–250. [Google Scholar]
- Bianchi, E.; Wright, G.J. Sperm Meets Egg: The Genetics of Mammalian Fertilization. Annu. Rev. Genet. 2016, 50, 93–111. [Google Scholar] [CrossRef]
- Just, E.E. The Biology of the Cell Surface; P. Blakiston’s Son & Co., Inc.: Philadelphia, PA, USA, 1939; pp. 1–392. [Google Scholar]
- Vasilev, F.; Limatola, N.; Chun, J.T.; Santella, L. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 2019, 15, 757–775. [Google Scholar] [CrossRef] [Green Version]
- Wessel, G.M.; Wong, J.L. Cell surface changes in the egg at fertilization. Mol. Reprod. Dev. 2009, 76, 942–953. [Google Scholar] [CrossRef] [Green Version]
- Just, E.E. The fertilization reaction in Echinarachnius parma. I. Cortical response of the egg to insemination. Biol. Bull. 1919, 36, 1–10. [Google Scholar] [CrossRef]
- Rothschild, L.; Swann, M.M. The Fertilization Reaction in the sea-urchin. J. Exp. Biol. 1952, 29, 469–483. [Google Scholar]
- Jaffe, L.A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 1976, 261, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Gould-Somero, M.; Jaffe, L.A.; Holland, L.Z. Electrically mediated fast polyspermy block in eggs of the marine worm, Urechis caupo. J. Cell Biol. 1979, 82, 426–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, S.; Hirai, S. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev. Biol. 1979, 70, 327–340. [Google Scholar] [CrossRef]
- Iwao, Y.; Izaki, K. Universality and diversity of a fast, electrical block to polyspermy during fertilization in animals. In Reproductive and Developmental Strategies; Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 499–533. [Google Scholar]
- Jaffe, L.A. The fast block to polyspermy: New insight into a century-old problem. J. Gen. Physiol. 2018, 150, 1233–1234. [Google Scholar] [CrossRef]
- DeFelice, L.J.; Dale, B. Voltage response to fertilization and polyspermy in sea urchin eggs and oocytes. Dev. Biol. 1979, 72, 327–341. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Grey, R.D. Controversy over the fast, partial, temporary block to polyspermy in sea urchins: A reevaluation. Dev. Biol. 1984, 103, 1–17. [Google Scholar] [CrossRef]
- Dale, B. Is the idea of a fast block to polyspermy based on artifact? Biochem. Biophys. Res. Commun. 2014, 450, 1159–1165. [Google Scholar] [CrossRef]
- Hertwig, O.; Hertwig, R. Über den befruchtungs- und teilungsvorgang des tierischen eies unter dem einflufs äurserer Agentien. JENA Z. Naturw. 1887, 20, 120–241. [Google Scholar]
- Clark, J.M. An experimental study of polyspermy. Biol. Bull. 1936, 70, 361–384. [Google Scholar] [CrossRef]
- Jaffe, L.A. Electrical polyspermy block in sea urchins: Nicotine and low sodium experiments. Dev. Growth Diff. 1980, 22, 503–507. [Google Scholar] [CrossRef]
- Dale, B.; de Santis, A.; Hagström, B.E. The effect of nicotine on sperm-Egg interaction in the sea urchin: Polyspermy and electrical events. Gamete Res. 1982, 5, 125–135. [Google Scholar] [CrossRef]
- McGehee, D.S.; Role, L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995, 57, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, G.; Vijayaraghavan, S. Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 2002, 53, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef] [Green Version]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Ivonnet, P.I.; Chambers, E.L. Nicotinic acetylcholine receptors of the neuronal type occur in the plasma membrane of sea urchin eggs. Zygote 1997, 5, 277–287. [Google Scholar] [CrossRef]
- Puppo, A.; Chun, J.T.; Gragnaniello, G.; Garante, E.; Santella, L. Alteration of the cortical actin cytoskeleton deregulates Ca2+ signaling, monospermic fertilization, and sperm entry. PLoS ONE 2008, 3, e3588. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.T.; Vasilev, F.; Santella, L. Antibody against the actin-binding protein depactin attenuates Ca2+ signaling in starfish eggs. Biochem. Biophys. Res. Commun. 2013, 441, 301–307. [Google Scholar] [CrossRef]
- Chun, J.T.; Limatola, N.; Vasilev, F.; Santella, L. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem. Biophys. Res. Commun. 2014, 450, 1166–1167. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Chun, J.T. Calcium and actin in the saga of awakening oocytes. Biochem. Biophys. Res. Commun. 2015, 460, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Limatola, N.; Vasilev, F.; Chun, J.T. Maturation and fertilization of echinoderm eggs: Role of actin cytoskeleton dynamics. Biochem. Biophys. Res. Commun. 2018, 506, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.; Swann, M.M. The fertilization reaction in the Sea-Urchin egg. The effect of nicotine. J. Exp. Biol. 1950, 27, 400–406. [Google Scholar] [PubMed]
- Kyerematen, G.A.; Vesell, E.S. Metabolism of nicotine. Drug Metab. Rev. 1991, 23, 3–41. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.F.; Presley, R. Promotion of polyspermy in the sea-urchin egg by nicotine and its antagonism by curare. J. Physiol. 1971, 213, 62P–63P. [Google Scholar]
- Ochoa, E.L.; Chattopadhyay, A.; McNamee, M.G. Desensitization of the nicotinic acetylcholine receptor: Molecular mechanisms and effect of modulators. Cell Mol. Neurobiol. 1989, 9, 141–178. [Google Scholar] [CrossRef]
- Hagström, B.E.; Allen, R.D. The mechanism of nicotine-induced polyspermy. Exp. Cell Res. 1956, 10, 14–23. [Google Scholar] [CrossRef]
- Lönning, S. Electron microscopic studies of the block to polyspermy - The influence of nicotine. Sarsia 1965, 18, 17–23. [Google Scholar] [CrossRef]
- Kyozuka, K.; Chun, J.T.; Puppo, A.; Gragnaniello, G.; Garante, E.; Santella, L. Guanine nucleotides in the meiotic maturation of starfish oocytes: Regulation of the actin cytoskeleton and of Ca2+ signaling. PLoS ONE 2009, 4, e6296. [Google Scholar] [CrossRef]
- Chun, J.T.; Puppo, A.; Vasilev, F.; Gragnaniello, G.; Garante, E.; Santella, L. The biphasic increase of PIP2 in the fertilized eggs of starfish: New roles in actin polymerization and Ca2+ signaling. PLoS ONE 2010, 5, e14100. [Google Scholar] [CrossRef] [Green Version]
- Burgess, D.R.; Schroeder, T.E. Polarized bundles of actin filaments within microvilli of fertilized sea urchin eggs. J. Cell Biol. 1977, 74, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Partin, J.S.; Stears, R.L.; Lennarz, W.J. Developmental expression of the sea urchin egg receptor for sperm. Dev. Biol. 1994, 165, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I. Nicotine to Nicotinoids: 1962 to 1997. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 3–28. [Google Scholar]
- Le Houezec, J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: A review. Int. J. Tuberc. Lung Dis. 2003, 7, 811–819. [Google Scholar] [PubMed]
- Fabian-Fine, R.; Skehel, P.; Errington, M.L.; Davies, H.A.; Sher, E.; Stewart, M.G.; Fine, A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001, 21, 7993–8003. [Google Scholar] [CrossRef]
- Oriol-Audit, C. Polyamine-induced actin polymerization. Eur. J. Biochem. 1978, 87, 371–376. [Google Scholar] [CrossRef]
- Kouyama, T.; Mihashi, K. Fluorimetry study of N-(1-pyrenyl) iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 1981, 114, 33–38. [Google Scholar] [CrossRef]
- Baldwin, I.T. Methyl jasmonate-induced nicotine production in Nicotiana attenuata: Inducing defenses in the field without wounding. Entomol. Exp. Appl. 1996, 80, 213–220. [Google Scholar] [CrossRef]
- Ujváry, I. Nicotine and Other Insecticidal Alkaloids. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 29–70. [Google Scholar]
- Rothschild, L. The fertilization reaction in the sea-urchin - The induction of polyspermy by nicotine. J. Exp. Biol. 1953, 30, 57–67. [Google Scholar]
- Vacquier, V.D.; Tegner, M.J.; Epel, D. Protease released from sea urchin eggs at fertilization alters the vitelline layer and aids in preventing polyspermy. Exp. Cell Res. 1973, 80, 111–119. [Google Scholar] [CrossRef]
- Chun, J.T.; Santella, L. The actin cytoskeleton in meiotic maturation and fertilization of starfish eggs. Biochem. Biophys. Res. Commun. 2009, 384, 141–143. [Google Scholar] [CrossRef]
- McCulloh, D.H.; Lynn, J.W.; Chambers, E.L. Membrane depolarization facilitates sperm entry, large fertilization cone formation, and prolonged current responses in sea urchin oocytes. Dev. Biol. 1987, 124, 177–190. [Google Scholar] [CrossRef]
- Shen, S.S.; Steinhardt, R.A. Time and voltage windows for reversing the electrical block to fertilization. Proc. Natl. Acad. Sci. USA 1984, 81, 1436–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019, 27, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Dale, B. New experimental data refuting the idea of a fast electrical block to polyspermy in sea urchin eggs. Zygote 2019, 27, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Altered actin cytoskeleton in ageing eggs of starfish affects fertilization process. Exp. Cell Res. 2019, 381, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Lange, K. Fundamental role of microvilli in the main functions of differentiated cells: Outline of an universal regulating and signaling system at the cell periphery. J. Cell Physiol. 2011, 226, 896–927. [Google Scholar] [CrossRef] [PubMed]
- Abood, L.G.; Latham, W.; Grassi, S. Isolation of a nicotine binding site from rat brain by affinity chromatography. Proc. Natl. Acad. Sci. USA 1983, 80, 536–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheek, T.R.; Burgoyne, R.D. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 1986, 207, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.L.; Rodríguez Del Castillo, A.; Tchakarov, L.; Trifaró, J.M. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J. Cell Biol. 1991, 113, 1057–1067. [Google Scholar] [CrossRef]
- Tchakarov, L.E.; Zhang, L.; Rosé, S.D.; Tang, R.; Trifaró, J.M. Light and electron microscopic study of changes in the organization of the cortical actin cytoskeleton during chromaffin cell secretion. J. Histochem. Cytochem. 1998, 46, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.I. Nicotine effects on neutrophil F-actin formation and calcium release: Implications for tobacco use and pulmonary diseases. Exp. Lung Res. 1994, 20, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Cucina, A.; Sapienza, P.; Borrelli, V.; Corvino, V.; Foresi, G.; Randone, B.; Cavallaro, A.; Santoro-D’Angelo, L. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. J. Surg. Res. 2000, 92, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, S.H.; Jong, A.; Zhou, Z.Y.; Li, W.; Suzuki, K.; Huang, S.H. Enhanced Escherichia coli invasion of human brain microvascular endothelial cells is associated with alternations in cytoskeleton induced by nicotine. Cell Microbiol. 2002, 4, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gong, H.; Wang, Y.; Wang, Y.; Yin, H.; Chen, P.; Zhang, H.; Wang, Y. Nicotine alters morphology and function of retinal pigment epithelial cells in mice. Toxicol. Pathol. 2010, 38, 560–567. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanabe, N.; Kawato, T.; Nakai, K.; Kariya, T.; Matsumoto, S.; Zhao, N.; Motohashi, M.; Maeno, M. Nicotine affects bone resorption and suppresses the expression of cathepsin K, MMP-9 and vacuolar-type H+-ATPase d2 and actin organization in osteoclasts. PLoS ONE 2013, 8, e59402. [Google Scholar] [CrossRef]
- Pomorski, P.; Wasik, A.; Kolodziejczyk, J.; Grebecka, L.; Klopocka, W. Nicotine affects behaviour, morphology and cortical cytoskeleton of Amoeba proteus. Acta Protozool. 2004, 43, 193–198. [Google Scholar]
- Nusco, G.A.; Chun, J.T.; Ercolano, E.; Lim, D.; Gragnaniello, G.; Kyozuka, K.; Santella, L. Modulation of calcium signalling by the actin-binding protein cofilin. Biochem. Biophys. Res. Commun. 2006, 348, 109–114. [Google Scholar] [CrossRef]
- Ziomek, C.A.; Epel, D. Polyspermy block of Spisula eggs is prevented by cytochalasin B. Science 1975, 189, 139–141. [Google Scholar] [CrossRef]
- Izadi, M.; Hou, W.; Qualmann, B.; Kessels, M.M. Direct effects of Ca2+/calmodulin on actin filament formation. Biochem. Biophys. Res. Commun. 2018, 506, 355–360. [Google Scholar] [CrossRef]
- Lange, K. Microvillar Ca++ signaling: A new view of an old problem. J. Cell Physiol. 1999, 180, 19–34. [Google Scholar] [CrossRef]
- Lim, D.; Lange, K.; Santella, L. Activation of oocytes by latrunculin A. FASEB J. 2002, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, F.; Limatola, N.; Park, D.R.; Kim, U.H.; Santella, L.; Chun, J.T. Disassembly of Subplasmalemmal Actin Filaments Induces Cytosolic Ca2+ Increases in Astropecten aranciacus Eggs. Cell. Physiol. Biochem. 2018, 48, 2011–2034. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.; Stein, Z.A.; Susser, M.; Warburton, D. Smoking: A risk factor for spontaneous abortion. N. Engl. J. Med. 1977, 297, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Hannibal, C.G.; Lindekilde, B.E.; Tolstrup, J.; Frederiksen, K.; Munk, C.; Bergholt, T.; Buss, L.; Ottesen, B.; Grønbaek, M.; et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet. Gynecol. Scand. 2006, 85, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.P.; White, K.L.; Rickords, L.F.; Sessions, B.R.; Aston, K.I.; Bunch, T.D. Nicotine alters bovine oocyte meiosis and affects subsequent embryonic development. Mol. Reprod. Dev. 2007, 74, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.P.; Sessions, B.R.; Rickords, L.F.; White, K.L.; Bunch, T.D. Nicotine induces multinuclear formation and causes aberrant embryonic development in bovine. Mol. Reprod. Dev. 2008, 75, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.A.; Jarvis, M.; Iyer, R.; Feyerabend, C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br. Med. J. 1980, 280, 972–976. [Google Scholar] [CrossRef] [Green Version]










| Nicotine (mM) | 0 | 0.1 | 0.2 | 0.5 | 1 | 1.5 | 2 | 6 | 20 |
| Egg-incorporated Sperm Count | 1.11 ± 0.33 | 1.14 ± 0.40 | 1.14 ± 0.40 | 1.23 ± 0.50 | 1.87 ± 1.07 | 2.2 ± 1.2 | 5.0 ± 4.0 * | 7.2 ± 2.5 * | 11.1 ± 3.9 * |
| Eggs with Full/Partial FE elevation | 100%/0% | 50%/18% | 24%/22% | 0%/33% | 0%/13% | 3.3%/6.7% | 0%/0% | 0%/0% | 0%/0% |
| n | 160 | 50 | 50 | 30 | 30 | 30 | 80 | 40 | 40 |
| Cotinine (mM) | 0 | 0.1 | 0.2 | 0.5 | 1 | 1.5 | 2 | 6 | 20 |
| Egg-incorporated Sperm Count | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.05 ± 0.22 | 1.1 ± 0.31 | 1.0 ± 0.0 | 1.05 ± 0.22 | 1.0 ± 0.0 | 1.1 ± 0.31 | 0.55 ± 0.60 # |
| Eggs with Full/Partial FE elevation | 100%/0% | 75%/25% | 65%/35% | 25%/45% | 5%/75% | 10%/20% | 0%/0% | 0%/0% | 0%/0% |
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Acetylcholine (mM) | 0 | 0.01 | 0.1 | 0.2 | 0.5 | 1 | 2 | 6 | 10 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Egg-incorporated sperm Count | 1.10 ± 0.31 | 1.10 ± 0.31 | 1.05 ± 0.22 | 1.05 ± 0.22 | 1.00 ± 0.0 | 1.05 ± 0.22 | 1.00 ± 0.0 | 1.00 ± 0.0 | 1.05 ± 0.22 | 1.00 ± 0.0 |
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limatola, N.; Vasilev, F.; Santella, L.; Chun, J.T. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells 2020, 9, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9010063
Limatola N, Vasilev F, Santella L, Chun JT. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells. 2020; 9(1):63. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9010063
Chicago/Turabian StyleLimatola, Nunzia, Filip Vasilev, Luigia Santella, and Jong Tai Chun. 2020. "Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics" Cells 9, no. 1: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9010063