Inguinal Ring RNA Sequencing Reveals Downregulation of Muscular Genes Related to Scrotal Hernia in Pigs
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Sample Collection
2.2. Total RNA Extraction, Library Preparation and Sequencing
2.3. RNA-Sequencing Analyses
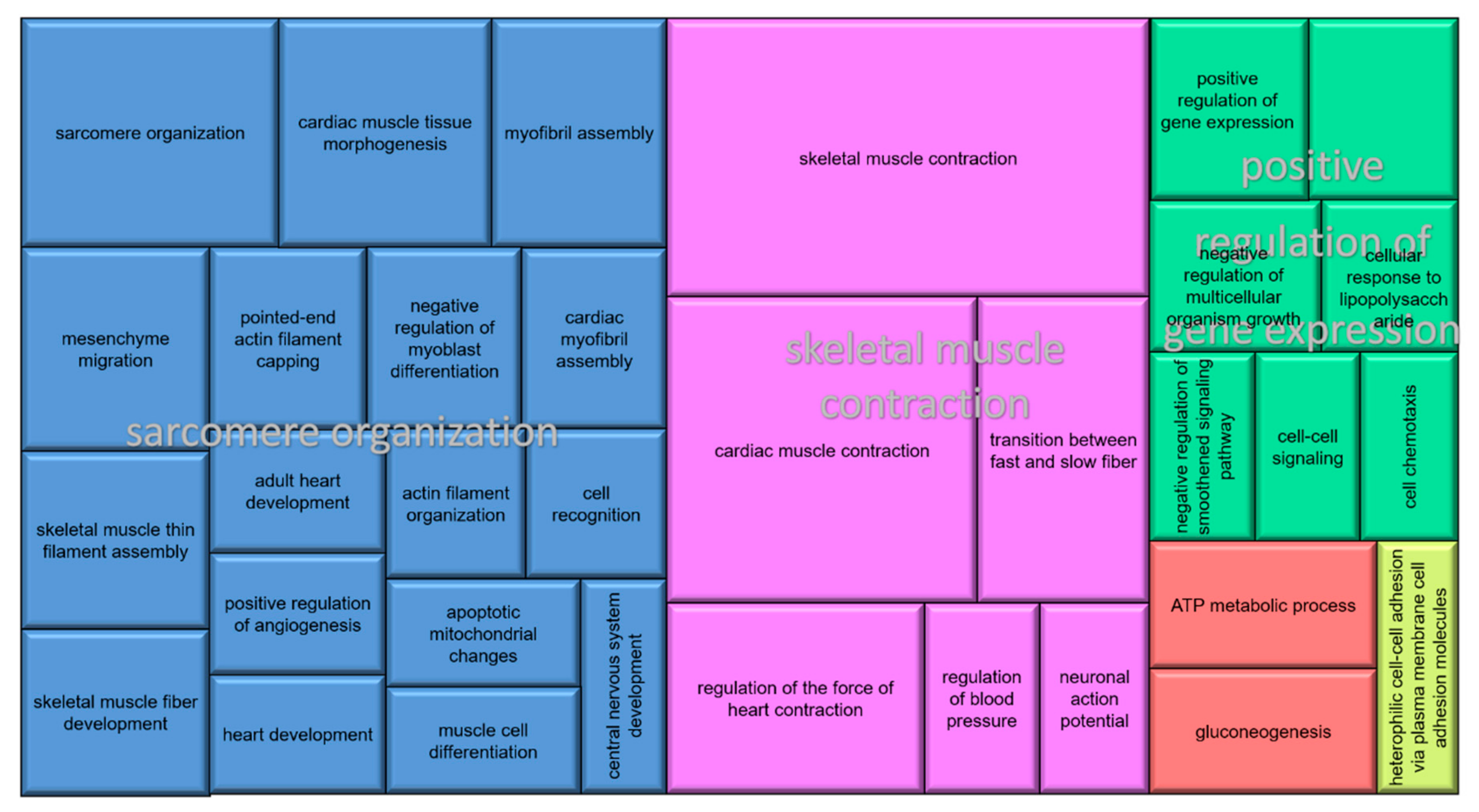
2.4. Functional Analysis
2.5. qPCR Confirmation
3. Results
3.1. Sequencing and Mapping
3.2. Differential Gene Expression
3.3. Functional Annotation and Pathway Analysis
4. Discussion
4.1. Prevalence of Patent Processus Vaginalis
4.2. Low Muscle Contractile Function in the Inguinal Region
4.3. Changes in Collagen Proportions in the Inguinal Ring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
References
- Derner, J.D.; Hunt, L.; Filho, K.E.; Ritten, J.; Capper, J.; Han, G. Livestock Production Systems; Springer: Berlin, Germany, 2017; pp. 347–372. [Google Scholar]
- Mattsson, P. Prevalence of Congenital Defects in Swedish Hampshire, Landrace and Yorkshire Pig Breeds and Opinions on Their Prevalence in Swedish Commercial Herds. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2011. [Google Scholar]
- Sevillano, C.A.; Lopes, M.S.; Harlizius, B.; Hanenberg, E.H.A.T.; Knol, E.F.; Bastiaansen, J.W.M. Genome-wide association study using deregressed breeding values for cryptorchidism and scrotal/inguinal hernia in two pig lines. Genet. Sel. Evol. 2015, 47, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elansary, M.; Stinckens, A.; Ahariz, N.; Cambisano, N.; Coppieters, W.; Grindflek, E.; van Son, M.; Buys, N.; Georges, M. On the use of the transmission disequilibrium test to detect pseudo-autosomal variants affecting traits with sex-limited expression. Anim. Genet. 2015, 46, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Grindflek, E.; Moe, M.; Taubert, H.; Simianer, H.; Lien, S.; Moen, T. Genome-wide linkage analysis of inguinal hernia in pigs using affected sib pairs. BMC Genet. 2006, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, J.; Bornemann-Kolatzki, K.; Knorr, C.; Taeubert, H.; Brenig, B. Molecular characterization and exclusion of porcine GUSB as a candidate gene for congenital hernia inguinalis/scrotalis. BMC Vet. Res. 2006, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Abrahamson, J. Etiology and pathophysiology of primary and recurrent groin hernia formation. Surg. Clin. N. Am. 1998, 78, 953–972. [Google Scholar] [CrossRef]
- Öberg, S.; Andresen, K.; Rosenberg, J. Etiology of Inguinal Hernias: A Comprehensive Review. Front. Surg. 2017, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Tanyel, F.C. Obliteration of processus vaginalis: Aberrations in the regulatory mechanism result in an inguinal hernia, hydrocele or undescended testis. Turk. J. Pediatr. 2004, 46, 18–27. [Google Scholar]
- Rosch, R.; Junge, K.; Lynen, P.; Mertens, P.R.; Klinge, U.; Schumpelick, V. Hernia—A Collagen Disease? Eur. Surg. Acta Chir. Austriaca 2003, 35, 11–15. [Google Scholar] [CrossRef]
- Du, Z.Q.; Zhao, X.; Vukasinovic, N.; Rodriguez, F.; Clutter, A.C.; Rothschild, M.F. Association and haplotype analyses of positional candidate genes in five genomic regions linked to scrotal hernia in commercial pig lines. PLoS ONE 2009, 4, e4837. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Du, Z.; Vukasinovic, N.; Rodriguez, F.; Clutter, A.C.; Rothschild, M.F. Association of HOXA10, ZFPM2, and MMP2 genes with scrotal hernias evaluated via biological candidate gene analyses in pigs. Am. J. Vet. Res. 2009, 70, 1006–1012. [Google Scholar] [CrossRef]
- Manalaysay, J.G.; Antonio, N.D.; Apilado, R.L.R.; Bambico, J.F.; Mingala, C.N. Screening of BCL-2 associated X protein gene polymorphism associated with scrotal hernia in domesticated swine using polymerase chain reaction-restriction fragment length polymorphism. Asian-Australas. J. Anim. Sci. 2017, 30, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Cookson, W.; Liang, L.; Abecasis, G.; Moffatt, M.; Lathrop, M. Mapping complex disease traits with global gene expression. Nat. Rev. Genet. 2009, 10, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Te Pas, M.; Madsen, O.; Calus, M.; Smits, M. The Importance of Endophenotypes to Evaluate the Relationship between Genotype and External Phenotype. Int. J. Mol. Sci. 2017, 18, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhbannikov, I.Y.; Arbeev, K.G.; Yashin, A.I. rqt: An R package for gene-level meta-analysis. Bioinformatics 2017, 33, 3129–3130. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Ostell, J.; Pruitt, K.D.; Karsch-Mizrachi, I. GenBank. Nucleic Acids Res. 2019, 47, D94–D99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Biosoft, P. NetPrimer. Available online: http://www.premierbiosoft.com/netprimer/index.html (accessed on 3 April 2019).
- Pfaffl, M.W. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Lorenzetti, W.R.; Ibelli, A.M.G.; De Oliveira Peixoto, J.; Mores, M.A.Z.; Savoldi, I.R.; Do Carmo, K.B.; De Oliveira, H.C.; Ledur, M.C. Identification of endogenous normalizing genes for expression studies in inguinal ring tissue for scrotal hernias in pigs. PLoS ONE 2018, 13, e0204348. [Google Scholar] [CrossRef]
- Ding, N.S.; Mao, H.R.; Guo, Y.M.; Ren, J.; Xiao, S.J.; Wu, G.Z.; Shen, H.Q.; Wu, L.H.; Ruan, G.F.; Brenig, B.; et al. A genome-wide scan reveals candidate susceptibility loci for pig hernias in an intercross between White Duroc and Erhualian. J. Anim. Sci. 2009, 87, 2469–2474. [Google Scholar] [CrossRef]
- Beuermann, C.; Beck, J.; Schmelz, U.; Dunkelberg, H.; Schütz, E.; Brenig, B.; Knorr, C. Tissue Calcium Content in Piglets with Inguinal or Scrotal Hernias or Cryptorchidism. J. Comp. Pathol. 2009, 140, 182–186. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa, A.; Romano, G.; Salamone, G.; Cocorullo, G.; Mularo, S.A.; Marasa, S.; Gulotta, G. Histological findings in direct inguinal hernia. Hernia 2013, 17, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Amato, G.; Agrusa, A.; Romano, G.; Salamone, G.; Gulotta, G.; Silvestri, F.; Bussani, R. Muscle degeneration in inguinal hernia specimens. Hernia 2012, 16, 327–331. [Google Scholar] [CrossRef]
- Antunes, I.F.; Haisma, H.J.; Elsinga, P.H.; Van Waarde, A.; Willemsen, A.T.M.; Dierckx, R.A.; De Vries, E.F.J. In vivo evaluation of 1-O-(4-(2-fluoroethyl-carbamoyloxymethyl)-2-nitrophenyl)-O-β-D-glucopyronuronate: A positron emission tomographic tracer for imaging β-Glucuronidase activity in a tumor/inflammation rodent model. Mol. Imaging 2012, 11, 77–87. [Google Scholar] [CrossRef]
- Naz, H.; Islam, A.; Waheed, A.; Sly, W.S.; Ahmad, F.; Hassan, M.I. Human β-Glucuronidase: Structure, Function, and Application in Enzyme Replacement Therapy. Rejuvenation Res. 2013, 16, 352–363. [Google Scholar] [CrossRef]
- Bauer, M.K.A.; Schubert, A.; Rocks, O.; Grimm, S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J. Cell Biol. 1999, 147, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2013, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Asada, A.; Takeshita, S.; Enomoto, M.; Yamakawa, E.; Tsutsumi, K.; Saito, T.; Satoh, T.; Itoh, H.; Kaziro, Y.; et al. Apoptosis-associated tyrosine kinase is a Cdk5 activator p35 binding protein. Biochem. Biophys. Res. Commun. 2003, 310, 398–404. [Google Scholar] [CrossRef]
- Ma, S.; Rubin, B.P. Apoptosis-Associated tyrosine kinase 1 inhibits growth and migration and promotes apoptosis in melanoma. Lab. Investig. 2014, 94, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Li, T.; Xu, D.C.; Liu, J.; Mao, G.; Cui, M.-Z.; Fu, X.; Xu, X. Death receptor 6 induces apoptosis not through type I or type II pathways, but via a unique mitochondria-dependent pathway by interacting with Bax protein. J. Biol. Chem. 2012, 287, 29125–29133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hosgor, M.; Karaca, I.; Ozer, E.; Erdag, G.; Ulukus, C.; Fescekoglu, O.; Aikawa, M. The role of smooth muscle cell differentiation in the mechanism of obliteration of processus vaginalis. J. Pediatr. Surg. 2004, 39, 1018–1023. [Google Scholar] [CrossRef]
- Kurpinski, K.; Lam, H.; Chu, J.; Wang, A.; Kim, A.; Tsay, E.; Agrawal, S.; Schaffer, D.V.; Li, S. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010, 28, 734–742. [Google Scholar] [CrossRef]
- Miano, J.M.; Cserjesi, P.; Ligon, K.L.; Periasamy, M.; Olson, E.N. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ. Res. 1994, 75, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rovere, R.M.L.; Roest, G.; Bultynck, G.; Parys, J.B. Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 2016, 60, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Bano, D.; Manni, S.; Rizzuto, R.; Carafoli, E. Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca(2+) signalling. EMBO J. 2000, 19, 4926–4935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuto, R.; Pinton, P.; Ferrari, D.; Chami, M.; Szabadkai, G.; Magalhães, P.J.; Di Virgilio, F.; Pozzan, T. Calcium and apoptosis: Facts and hypotheses. Oncogene 2003, 22, 8619–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)-Structure, function, and regulation in health and disease. Mol. Asp. Med. 2013, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Nyhus, L.M.; Klein, M.S.; Rogers, F.B. Inguinal hernia. Curr. Probl. Surg. 1991, 28, 407–450. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharm. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [Green Version]
- Knöll, R. Myosin binding protein C: Implications for signal-transduction. J. Muscle Res. Cell Motil. 2012, 33, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Ghahramani Seno, M.M.; Trollet, C.; Athanasopoulos, T.; Graham, I.R.; Hu, P.; Dickson, G. Transcriptomic analysis of dystrophin RNAi knockdown reveals a central role for dystrophin in muscle differentiation and contractile apparatus organization. BMC Genom. 2010, 11, 345. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Govindan, S.; Lee, K.; Zhao, P.; Han, R.; Runte, K.E.; Craig, R.; Palmer, B.M.; Sadayappan, S. Cardiac Myosin Binding Protein-C Plays No Regulatory Role in Skeletal Muscle Structure and Function. PLoS ONE 2013, 8, e69671. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, F.; Tander, B.; Baysal, K.; Bernay, F. High association of congenital heart disease with indirect inguinal hernia. Pediatr. Cardiol. 2005, 26, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A. Molecular genetics and pathogenesis of cardiomyopathy. J. Hum. Genet. 2016, 61, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.G.; Seidman, C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell 2001, 104, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Hutson, J.M.; Hasthorpe, S. Testicular descent and cryptorchidism: The state of the art in 2004. J. Pediatr. Surg. 2005, 40, 297–302. [Google Scholar] [CrossRef]
- Bendavid, R. The Unified Theory of hernia formation. Hernia 2004, 8, 171–176. [Google Scholar] [CrossRef]
- Henriksen, N.A.; Yadete, D.H.; Sorensen, L.T.; Ågren, M.S.; Jorgensen, L.N. Connective tissue alteration in abdominal wall hernia. Br. J. Surg. 2011, 98, 210–219. [Google Scholar] [CrossRef]
- Spivey, K.A.; Chung, I.; Banyard, J.; Adini, I.; Feldman, H.A.; Zetter, B.R. A role for collagen XXIII in cancer cell adhesion, anchorage-independence and metastasis. Oncogene 2012, 31, 2362–2372. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Yomogida, K.; Wada, T.; Yorihuzi, T.; Nishimune, Y.; Hosokawa, N.; Nagata, K. Type XXVI collagen, a new member of the collagen family, is specifically expressed in the testis and ovary. J. Biol. Chem. 2002, 277, 37678–37684. [Google Scholar] [CrossRef] [Green Version]
- Becerril, C.; Pardo, A.; Montaño, M.; Ramos, C.; Ramírez, R.; Selman, M. Acidic fibroblast growth factor induces an antifibrogenic phenotype in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Tanyel, F.C.; Müftüoglu, S.; Dagdeviren, A.; Kaymaz, F.F.; Büyükpamukçu, N. Myofibroblasts defined by electron microscopy suggest the dedifferentiation of smooth muscle within the sac walls associated with congenital inguinal hernia. BJU Int. 2001, 87, 251–255. [Google Scholar] [CrossRef]
- Shimbori, C.; Bellaye, P.S.; Xia, J.; Gauldie, J.; Ask, K.; Ramos, C.; Becerril, C.; Pardo, A.; Selman, M.; Kolb, M. Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J. Pathol. 2016, 240, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Chiego, D.J. Essentials of Oral Histology and Embryology; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2014; Volume 58, ISBN 9788578110796. [Google Scholar]

| Gene | Primer Sequence | Ensembl ID |
|---|---|---|
| MYBPC1 | F: CAAAAGGGGAGGCTGGAACT | ENSSSCG00000000866 |
| R: GCCCGACTACTCAAACCTGG | ||
| DES | F: ACTTCCGAGAAACAAGCCCT | ENSSSCG00000020785 |
| R: TGGCTTTAGAGCACCTCGTG | ||
| ACTA1 | F: TGAAGATCAAGATCATCGCCCC | ENSSSCG00000010190 |
| R: CAGCTGTTGGAATGGGGTTTAG | ||
| ACTG2 | F: CCTTCATCGGCATGGAGTCAG | ENSSSCG00000008294 |
| R: CAGCTGTTGGAATGGGGTTTAG | ||
| MAP1LC3C | F: TGGAAACAGCTGGAGGAATGAG | ENSSSCG00000010870 |
| R: CCTCTCTTCTGGTTGCTAAGCTC | ||
| GUSB | F: GACGGACACCTCCAAGTACC | ENSSSCG00000007739 |
| R: CAGTCCCGCGTAGTTGAAGAA | ||
| CNN1 | F: TGAGGTCAAGAACAAGCTGGC | ENSSSCG00000013614 |
| R: GGGTGGACTCATTGACCTTCTTC | ||
| FGF1 | F: CAGTGACAGCACAGAGCAGA | ENSSSCG00000024954 |
| R: GGTGCTTTCGAGGCTGAAGA |
| Downregulated Genes | ||||
|---|---|---|---|---|
| Ensembl Gene ID | Gene Symbol | Description | LogFC | FDR |
| ENSSSCG00000010190 | ACTA1 | Actin, α skeletal muscle | −13.41 | 4.80 × 10−8 |
| ENSSSCG00000000866 | MYBPC1 | Myosin binding protein C, slow type | −13.28 | 2.95 × 10−10 |
| ENSSSCG00000016157 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | −12.91 | 4.82 × 10−8 |
| ENSSSCG00000032720 | SMPX | Small muscular protein | −12.25 | 4.94 × 10−7 |
| ENSSSCG00000014324 | MYOT | Myotilin | −11.85 | 2.27 × 10−7 |
| ENSSSCG00000031903 | TNNT3 | Troponin T, fast skeletal muscle | −11.61 | 1.28 × 10−7 |
| ENSSSCG00000039710 | MYL2 | Myosin regulatory light chain 2, Ventricular/cardiac muscle isoform | −11.33 | 1.33 × 10−8 |
| ENSSSCG00000013354 | CSRP3 | Cysteine and glycine-rich protein 3 | −11.24 | 6.61 × 10−7 |
| ENSSSCG00000011325 | MYL3 | Myosin light chain 3 | −10.96 | 5.96 × 10−8 |
| ENSSSCG00000029441 | MYH2 | Myosin-2 | −10.78 | 1.59 × 10−6 |
| Upregulated Genes | ||||
| Ensembl Gene ID | Gene Symbol | Description | logFC | FDR |
| ENSSSCG00000029289 | Uncharacterized | 7.13 | 1.51 × 10−23 | |
| ENSSSCG00000034838 | MAP1LC3C | Microtubule associated protein 1 light chain 3 γ | 6.72 | 1.96 × 10−26 |
| ENSSSCG00000039102 | Uncharacterized | 5.01 | 1.04 × 10−7 | |
| ENSSSCG00000036318 | Uncharacterized | 4.70 | 4.15 × 10−7 | |
| ENSSSCG00000036983 | Uncharacterized | 4.67 | 3.20 × 10−6 | |
| ENSSSCG00000039804 | Uncharacterized | 4.55 | 6.71 × 10−8 | |
| ENSSSCG00000007678 | COL26A1 | Collagen type XXVI α 1 chain | 4.54 | 4.37 × 10−10 |
| ENSSSCG00000038719 | Uncharacterized | 4.53 | 7.59 × 10−7 | |
| ENSSSCG00000036203 | Uncharacterized | 4.44 | 2.62 × 10−6 | |
| ENSSSCG00000039111 | Uncharacterized | 4.42 | 3.48 × 10−8 | |
| RNA-Seq | qPCR | |||
|---|---|---|---|---|
| Gene | LogFC | FDR | LogFC | p-Value |
| MYBPC1 | −13.28 | 2.95 × 10−10 | −12.29 | 0.000 |
| ACTA1 | −13.41 | 4.80 × 10−8 | −13.29 | 0.105 |
| ACTG2 | −5.95 | 9.12 × 10−8 | −5.97 | 0.006 |
| CNN1 | −3.73 | 4.16 × 10−8 | −3.68 | 0.023 |
| DES | −7.45 | 6.09 × 10−14 | −7.38 | 0.005 |
| FGF1 | 1.91 | 3.74 × 10−3 | 1.65 | 0.067 |
| GUSB | 1.00 | 1.58 × 10−2 | 0.71 | 0.082 |
| MAP1LC3C | 6.72 | 1.96 × 10−26 | 8.27 | 0.023 |
| Ensembl Gene ID | Description | Length | e-Value | Sim Mean | logFC |
|---|---|---|---|---|---|
| ENSSSCG00000035429 | Hemojuvelin isoform X3 | 1706 | 5.00 × 10−119 | 96.77 | −9.70 |
| ENSSSCG00000034015 | Xin actin-binding repeat-containing protein 1 isoform X1 | 5511 | 0 | 87.7 | −9.49 |
| ENSSSCG00000036235 | Creatine kinase M-type | 252 | 1.52 × 10−17 | 100 | −8.78 |
| ENSSSCG00000015747 | Myomesin-2 | 7217 | 0 | 93.38 | −7.76 |
| ENSSSCG00000036052 | Titin isoform X1 | 1326 | 0 | 98.06 | −7.62 |
| ENSSSCG00000039804 | Immunoglobulin heavy chain variable region | 405 | 1.29 × 10−65 | 97.2 | 4.55 |
| ENSSSCG00000036983 | IgG heavy chain precursor | 987 | 0 | 96.07 | 4.67 |
| ENSSSCG00000036318 | IgG heavy chain precursor | 327 | 6.64 × 10−73 | 98.62 | 4.70 |
| ENSSSCG00000039102 | Immunoglobulin kappa Variable region | 297 | 5.24 × 10−63 | 94.09 | 5.01 |
| ENSSSCG00000029289 | Cystatin-9-like | 1146 | 1.71 × 10−93 | 76.56 | 7.13 |
| Canonical Pathway | Genes | p-Value | |
|---|---|---|---|
| ssc05410 | Hypertrophic cardiomyopathy (HCM) | TGFB3; TPM1; ACTC1; TPM2; MYL3; TNNC1; PRKAG3; CACNG1; CACNB1; SGCA; DES; CACNA1S | 4.16 × 10−6 |
| ssc05414 | Dilated cardiomyopathy | TGFB3; TPM1; ACTC1; TPM2; ADRB1; MYL3; TNNC1; CACNG1; CACNB1; SGCA; DES; CACNA1S | 7.72 × 10−6 |
| ssc04261 | Adrenergic signaling in cardiomyocytes | MYH7; TPM1; ACTC1; TPM2; ATP1A2; PLCB4; ADRA1A; ADRB1; MYL3; TNNC1; SCN7A; CACNG1; CACNB1; CACNA1S | 2.64 × 10−5 |
| ssc04022 | cGMP-PKG signaling pathway | SLC8A3; ATP1A2; PLCB4; MYLK2; ATP2A1; EDNRB; ADRA1A; PPIF; ADRB1; MYLK; SLC25A4; KCNMB1; MRVI1; ADORA1; CACNA1S | 5.02 × 10−5 |
| ssc04260 | Cardiac muscle contraction | MYH7; TPM1; ACTC1; TPM2; ATP1A2; MYL3; TNNC1; CACNG1; CACNB1; CACNA1S | 9.46 × 10−5 |
| ssc04152 | AMPK signaling pathway | LIPE; ADRA1A; FBP2; PFKFB3; PPARG; PRKAG3; PFKM; CPT1B; SLC2A4; LEPR; PPARGC1A | 8.12 × 10−4 |
| ssc04020 | Calcium signaling pathway | SLC8A3; PLCB4; MYLK2; TNNC2; ATP2A1; EDNRB; ADRA1A; PPIF; ADRB1; TNNC1; MYLK; SLC25A4; CACNA1S | 2.04 × 10−3 |
| ssc00250 | Alanine, aspartate and glutamate metabolism | ALDH5A1; GPT2; DDO; ABAT; ASPA | 1.31 × 10−2 |
| ssc04923 | Regulation of lipolysis in adipocytes | LIPE; ADRB1; ENSSSCG00000010992; PNPLA2; ADORA1; MGL | 1.51 × 10−2 |
| ssc01100 | Metabolic pathways | TST; PIK3C2G; ALDH5A1; ANPEP; FUT8; GPT2; AK5; PGM1; GALNT12; AK1; PHGDH; AMPD1; PLCB4; GUSB; ABAT; ST3GAL5; B3GNT2; LPIN1; CMPK2; HPGDS; HPSE; EPHX2; GAL3ST1; FBP2; ACAA1; PLD1; PNPLA2; PC; PYGM; CKMT2; P4HA3; CYP27A1; ATP6V0A4; PGAM2; ASPA; ENO3; PFKM; ; CKB; DGKG; PYGB; MGLL; ACSM5 | 1.81 × 10−2 |
| ssc04910 | Insulin signaling pathway | LIPE; FBP2; PYGM; PRKAG3; PPP1R3A; PPP1R3B; PYGB; SLC2A4; PPARGC1A | 1.93 × 10−2 |
| ssc01230 | Biosynthesis of amino acids | GPT2; PHGDH; PC; PGAM2; ENO3; PFKM | 1.98 × 10−2 |
| ssc04931 | Insulin resistance | PYGM; PRKAG3; PPP1R3A; CPT1B; PPP1R3B; PYGB; SLC2A4; PPARGC1A | 2.49 × 10−2 |
| ssc03320 | PPAR signaling pathway | OLR1; ENSSSCG00000010992; ACAA1; PPARG; CYP27A1; CPT1B | 2.85 × 10−2 |
| ssc04270 | Vascular smooth muscle contraction | PLCB4; MYLK2; ADRA1A; ACTA2; MYLK; KCNMB1; MRVI1; CACNA1S | 2.95 × 10−2 |
| ssc04922 | Glucagon signaling pathway | PLCB4; PYGM; PRKAG3; PGAM2; CPT1B; PYGB; PPARGC1A | 3.51 × 10−2 |
| ssc04921 | Oxytocin signaling pathway | PLCB4; MYLK2; MYLK; CAMK1G; PRKAG3; CACNG1; CACNB1; KCNJ5; CACNA1S | 3.91 × 10−2 |
| ssc01200 | Carbon metabolism | GPT2; PHGDH; FBP2; PC; PGAM2; ENO3; PFKM | 4.90 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.d.S.; Ibelli, A.M.G.; Lorenzetti, W.R.; Weber, T.; Peixoto, J.d.O.; Cantão, M.E.; Mores, M.A.Z.; Morés, N.; Pedrosa, V.B.; Coutinho, L.L.; et al. Inguinal Ring RNA Sequencing Reveals Downregulation of Muscular Genes Related to Scrotal Hernia in Pigs. Genes 2020, 11, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11020117
Romano GdS, Ibelli AMG, Lorenzetti WR, Weber T, Peixoto JdO, Cantão ME, Mores MAZ, Morés N, Pedrosa VB, Coutinho LL, et al. Inguinal Ring RNA Sequencing Reveals Downregulation of Muscular Genes Related to Scrotal Hernia in Pigs. Genes. 2020; 11(2):117. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11020117
Chicago/Turabian StyleRomano, Gabrieli de Souza, Adriana Mercia Guaratini Ibelli, William Raphael Lorenzetti, Tomás Weber, Jane de Oliveira Peixoto, Mauricio Egídio Cantão, Marcos Antônio Zanella Mores, Nelson Morés, Victor Breno Pedrosa, Luiz Lehmann Coutinho, and et al. 2020. "Inguinal Ring RNA Sequencing Reveals Downregulation of Muscular Genes Related to Scrotal Hernia in Pigs" Genes 11, no. 2: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11020117







