Genomic Organization and Generation of Genetic Variability in the RHS (Retrotransposon Hot Spot) Protein Multigene Family in Trypanosoma cruzi
Abstract
:1. Introduction
2. Material and Methods
2.1. Parasites
2.2. Identification of RHS Sequences in T. cruzi and T. cruzi marinkellei Genome Databases
2.3. Classification and Phylogenetic Analyses of RHS
2.4. Detection of Potential Recombination Events in RHS Sequences
2.5. Expression and Purification of Recombinant RHS
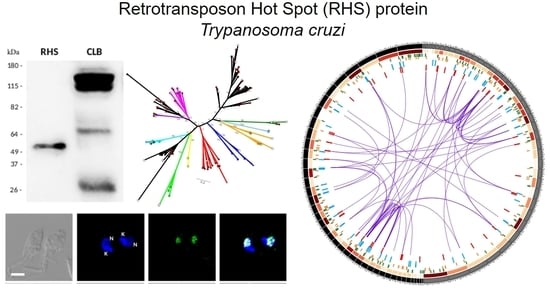
2.6. Antibody Production, Western Blot, and Immunofluorescence Analyses
3. Results
3.1. Mapping of RHS Sequences on the Chromosomes of Clone CLB of T. cruzi
3.2. Phylogeny and Classification of the RHS Multigene Family of Clone CLB
3.3. Generation of Genetic Variability by Recombination between T. cruzi RHS Sequences
3.4. Expression and Subcellular Localization of RHS in T. cruzi
4. Discussion
4.1. Genomic Organization and Generation of Genetic Variability in the RHS Multigene Family in T. cruzi
4.2. The Role of RHS Proteins in T. cruzi
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Chagas Disease (American Trypanosomiasis). 2020. Available online: https://www.who.int/health-topics/chagas-disease (accessed on 27 July 2020).
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The Genome Sequence of Trypanosoma cruzi, Etiologic Agent of Chagas Disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Berná, L.; Rodriguez, M.; Chiribao, M.L.; Parodi-Talice, A.; Pita, S.; Rijo, G.; Alvarez-Valin, F.; Robello, C. Expanding an expanded genome: Long-read sequencing of Trypanosoma cruzi. Microb. Genomics 2018, 4. [Google Scholar] [CrossRef]
- Pita, S.; Díaz-Viraqué, F.; Iraola, G.; Robello, C. The Tritryps Comparative Repeatome: Insights on Repetitive Element Evolution in Trypanosomatid Pathogens. Genome Biol. Evol. 2019, 11, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Chiurillo, M.A.; Barros, R.R.M.; Souza, R.T.; Marini, M.M.; Antonio, C.R.; Cortez, D.R.; Curto, M.; Lorenzi, H.A.; Schijman, A.G.; Ramirez, J.L.; et al. Subtelomeric I-SceI-mediated double-strand breaks are repaired by homologous recombination in Trypanosoma cruzi. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bringaud, F.; Biteau, N.; Melville, S.E.; Hez, S.; El-Sayed, N.M.; Leech, V.; Berriman, M.; Hall, N.; Donelson, J.E.; Baltz, T. A New, Expressed Multigene Family Containing a Hot Spot for Insertion of Retroelements Is Associated with Polymorphic Subtelomeric Regions of Trypanosoma brucei. Eukaryot. Cell 2002, 1, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Bringaud, F.; Bartholomeu, D.C.; Blandin, G.; Delcher, A.; Baltz, T.; El-Sayed, N.M.A.; Ghedin, E. The Trypanosoma cruzi L1Tc and NARTc Non-LTR Retrotransposons Show Relative Site Specificity for Insertion. Mol. Biol. Evol. 2006, 23, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Durand-Dubief, M.; Absalon, S.; Menzer, L.; Ngwabyt, S.; Ersfeld, K.; Bastin, P. The Argonaute protein TbAGO1 contributes to large and mini-chromosome segregation and is required for control of RIME retroposons and RHS pseudogene-associated transcripts. Mol. Biochem. Parasitol. 2007, 156, 144–153. [Google Scholar] [CrossRef]
- Wen, Y.-Z.; Zheng, L.-L.; Liao, J.-Y.; Wang, M.-H.; Wei, Y.; Guo, X.-M.; Qu, L.-H.; Ayala, F.J.; Lun, Z.-R. Pseudogene-derived small interference RNAs regulate gene expression in African Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2011, 108, 8345–8350. [Google Scholar] [CrossRef] [Green Version]
- Naguleswaran, A.; Gunasekera, K.; Schimanski, B.; Heller, M.; Hemphill, A.; Ochsenreiter, T.; Roditi, I. Trypanosoma brucei RRM1 Is a Nuclear RNA-Binding Protein and Modulator of Chromatin Structure. MBio 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Florini, F.; Naguleswaran, A.; Gharib, W.H.; Bringaud, F.; Roditi, I. Unexpected diversity in eukaryotic transcription revealed by the retrotransposon hotspot family of Trypanosoma brucei. Nucleic Acids Res. 2019, 47, 1725–1739. [Google Scholar] [CrossRef] [Green Version]
- Zingales, B.; Pereira, M.E.S.; Almeida, K.A.; Umezawa, E.S.; Nehme, N.S.; Oliveira, R.P.; Macedo, A.; Souto, R.P. Biological Parameters and Molecular Markers of Clone CL Brener—The Reference Organism of the Trypanosoma cruzi Genome Project. Mem. Inst. Oswaldo Cruz 1997, 92, 811–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, N. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect. Immun. 1983, 40, 836–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steindel, M.; Pinto, J.C.C.; Toma, H.K.; Mangia, R.H.R.; Ribeiro-Rodrigues, R.; Romanha, A.J. Trypanosoma rangeli (Tejera, 1920) isolated from a sylvatic rodent (Echimys dasythrix) in Santa Catarina island, Santa Catarina state: First report of this trypanosome in southern Brazil. Mem. Inst. Oswaldo Cruz 1991, 86, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, E.P. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar]
- Vieira da Silva, C.; Luquetti, A.O.; Rassi, A.; Mortara, R.A. Involvement of Ssp-4-related carbohydrate epitopes in mammalian cell invasion by Trypanosoma cruzi amastigotes. Microbes Infect. 2006, 8, 2120–2129. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Conserved Domains and Protein Classification. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/cdd (accessed on 10 February 2017).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [Green Version]
- TriTrypDB-31_TcruziCLBrener_AnnotatedTranscripts.fast. Available online: https://tritrypdb.org/common/downloads/release-31/TcruziCLBrener/fasta/data/ (accessed on 12 April 2017).
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- FigTree GitHub Repository. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 March 2017).
- Martins, N.O.; de Souza, R.T.; Cordero, E.M.; Maldonado, D.C.; Cortez, C.; Marini, M.M.; Ferreira, E.R.; Bayer-Santos, E.; de Almeida, I.C.; Yoshida, N.; et al. Molecular Characterization of a Novel Family of Trypanosoma cruzi Surface Membrane Proteins (TcSMP) Involved in Mammalian Host Cell Invasion. PLoS Negl. Trop. Dis. 2015, 9, e0004216. [Google Scholar] [CrossRef]
- Tibayrenc, M.; Ward, P.; Moya, A.; Ayala, F.J. Natural populations of Trypanosoma cruzi, the agent of Chagas disease, have a complex multiclonal structure. Proc. Natl. Acad. Sci. USA. 1986, 83, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.P.; Broude, N.E.; Macedo, A.M.; Cantor, C.R.; Smith, C.L.; Pena, S.D.J. Probing the genetic population structure of Trypanosoma cruzi with polymorphic microsatellites. Proc. Natl. Acad. Sci. USA 1998, 95, 3776–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibayrenc, M.; Ayala, F.J. The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol. 2002, 18, 405–410. [Google Scholar] [CrossRef]
- Pena, S.D.J.; Machado, C.R.; Macedo, A.M. Trypanosoma cruzi: Ancestral genomes and population structure. Mem. Inst. Oswaldo Cruz 2009, 104, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaunt, M.W.; Yeo, M.; Frame, I.A.; Stothard, J.R.; Carrasco, H.J.; Taylor, M.C.; Mena, S.S.; Veazey, P.; Miles, G.A.J.; Acosta, N.; et al. Mechanism of genetic exchange in American trypanosomes. Nature 2003, 421, 936–939. [Google Scholar] [CrossRef]
- Machado, C.A.; Ayala, F.J. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA 2001, 98, 7396–7401. [Google Scholar] [CrossRef] [Green Version]
- Augusto-Pinto, L.; Teixeira, S.M.R.; Pena, S.D.J.; Machado, C.R. Single-nucleotide polymorphisms of the Trypanosoma cruzi MSH2 gene support the existence of three phylogenetic lineages presenting differences in mismatch-repair efficiency. Genetics 2003, 164, 117–126. [Google Scholar]
- Brisse, S.; Henriksson, J.; Barnabé, C.; Douzery, E.J.P.; Berkvens, D.; Serrano, M.; De Carvalho, M.R.C.; Buck, G.A.; Dujardin, J.-C.; Tibayrenc, M. Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect. Genet. Evol. 2003, 2, 173–183. [Google Scholar] [CrossRef]
- Westenberger, S.J.; Barnabé, C.; Campbell, D.A.; Sturm, N.R. Two Hybridization Events Define the Population Structure of Trypanosoma cruzi. Genetics 2005, 171, 527–543. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, J.M.; Augusto-Pinto, L.; Pimenta, J.R.; Bastos-Rodrigues, L.; Gonçalves, V.F.; Teixeira, S.M.R.; Chiari, E.; Junqueira, Â.C.V.; Fernandes, O.; Macedo, A.M.; et al. Ancestral Genomes, Sex, and the Population Structure of Trypanosoma cruzi. PLoS Pathog. 2006, 2, e24. [Google Scholar] [CrossRef] [Green Version]
- Berry, A.S.F.; Salazar-Sánchez, R.; Castillo-Neyra, R.; Borrini-Mayorí, K.; Chipana-Ramos, C.; Vargas-Maquera, M.; Ancca-Juarez, J.; Náquira-Velarde, C.; Levy, M.Z.; Brisson, D. Sexual reproduction in a natural Trypanosoma cruzi population. PLoS Negl. Trop. Dis. 2019, 13, e0007392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabl, P.; Imamura, H.; Van den Broeck, F.; Costales, J.A.; Maiguashca-Sánchez, J.; Miles, M.A.; Andersson, B.; Grijalva, M.J.; Llewellyn, M.S. Meiotic sex in Chagas disease parasite Trypanosoma cruzi. Nat. Commun. 2019, 10, 3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibayrenc, M. Genetic subdivisions within Trypanosoma cruzi (Discrete Typing Units) and their relevance for molecular epidemiology and experimental evolution. Kinetoplastid Biol. Dis. 2003, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zingales, B.; Andrade, S.; Briones, M.; Campbell, D.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.; Machado, C.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.G.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Weatherly, D.B.; Boehlke, C.; Tarleton, R.L. Chromosome level assembly of the hybrid Trypanosoma cruzi genome. BMC Genom. 2009, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- TriTrypDB. The Kinetoplastid Genomics Resource. Available online: https://tritrypdb.org/tritrypdb/ (accessed on 15 February 2017).
- El-Sayed, N.M.; Myler, P.J.; Blandin, G.; Berriman, M.; Crabtree, J.; Aggarwal, G.; Caler, E.; Renauld, H.; Worthey, E.A.; Hertz-Fowler, C.; et al. Comparative genomics of trypanosomatid parasitic protozoa. Science 2005, 309, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Azuaje, F.J.; Ramirez, J.L.; Da Silveira, J.F. In silico, biologically-inspired modelling of genomic variation generation in surface proteins of Trypanosoma cruzi. Kinetoplastid Biol. Dis. 2007, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Callejas-Hernández, F.; Rastrojo, A.; Poveda, C.; Gironès, N.; Fresno, M. Genomic assemblies of newly sequenced Trypanosoma cruzi strains reveal new genomic expansion and greater complexity. Sci. Rep. 2018, 8, 14631. [Google Scholar] [CrossRef]
- Moraes Barros, R.R.; Marini, M.M.; Antônio, C.; Cortez, D.R.; Miyake, A.M.; Lima, F.M.; Ruiz, J.C.; Bartholomeu, D.C.; Chiurillo, M.A.; Ramirez, J.; et al. Anatomy and evolution of telomeric and subtelomeric regions in the human protozoan parasite Trypanosoma cruzi. BMC Genomics 2012, 13, 229. [Google Scholar] [CrossRef] [Green Version]
- Chiurillo, M.A.; Regina Antonio, C.; Mendes Marini, M.; de Souza, R.T.; Franco da Silveira, J. Chromosomes Ends and Telomere Biology of Trypanosomatids. In Frontiers in Parasitology; Bentham Science United Arab Emirates: Sharjah, UAE, 2017; pp. 104–133. [Google Scholar]
- Ghedin, E.; Bringaud, F.; Peterson, J.; Myler, P.; Berriman, M.; Ivens, A.; Andersson, B.; Bontempi, E.; Eisen, J.; Angiuoli, S.; et al. Gene synteny and evolution of genome architecture in trypanosomatids. Mol. Biochem. Parasitol. 2004, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chiurillo, M.A.; El-Sayed, N.; Jones, K.; Santos, M.R.M.; Porcile, P.E.; Andersson, B.; Myler, P.; da Silveira, J.F.; Ramírez, J.L. Telomere and subtelomere of Trypanosoma cruzi chromosomes are enriched in (pseudo)genes of retrotransposon hot spot and trans-sialidase-like gene families: The origins of T. cruzi telomeres. Gene 2005, 346, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeu, D.C.; Cerqueira, G.C.; Leão, A.C.A.; DaRocha, W.D.; Pais, F.S.; Macedo, C.; Djikeng, A.; Teixeira, S.M.R.; El-Sayed, N.M. Genomic organization and expression profile of the mucin-associated surface protein (masp) family of the human pathogen Trypanosoma cruzi. Nucleic Acids Res. 2009, 37, 3407–3417. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Baptista, C.S.; Ienne, S.; Cerqueira, G.C.; Bartholomeu, D.C.; Zingales, B. Genomic organization and transcription analysis of the 195-bp satellite DNA in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2008, 160, 60–64. [Google Scholar] [CrossRef]
- Ramirez, J.L. An Evolutionary View of Trypanosoma cruzi Telomeres. Front. Cell. Infect. Microbiol. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Zolan, M.E. Chromosome-length polymorphism in fungi. Microbiol. Rev. 1995, 59, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Symington, L.S.; Rothstein, R.; Lisby, M. Mechanisms and Regulation of Mitotic Recombination in Saccharomyces cerevisiae. Genetics 2014, 198, 795–835. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Júnior, A.D.C.M.; Kalume, D.E.; Camargo, R.; Gómez-Mendoza, D.P.; Correa, J.R.; Charneau, S.; de Sousa, M.V.; de Lima, B.D.; Ricart, C.A.O. Unveiling the Trypanosoma cruzi Nuclear Proteome. PLoS ONE 2015, 10, e0138667. [Google Scholar] [CrossRef] [Green Version]
- Thon, G.; Baltz, T.; Eisen, H. Antigenic diversity by the recombination of pseudogenes. Genes Dev. 1989, 3, 1247–1254. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.D.; Ginger, M.L.; Burton, P.; McCulloch, R. Why are parasite contingency genes often associated with telomeres? Int. J. Parasitol. 2003, 33, 29–45. [Google Scholar] [CrossRef]
- Marcello, L.; Barry, J.D. Analysis of the VSG gene silent archive in Trypanosoma brucei reveals that mosaic gene expression is prominent in antigenic variation and is favored by archive substructure. Genome Res. 2007, 17, 1344–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.P.J.; Wang, H.; Barry, J.D. Mosaic VSGs and the Scale of Trypanosoma brucei Antigenic Variation. PLoS Pathog. 2013, 9, e1003502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, C.; Bringaud, F.; Layden, R.E.; Baltz, T.; Eisen, H. Active late-appearing variable surface antigen genes in Trypanosoma equiperdum are constructed entirely from pseudogenes. Proc. Natl. Acad. Sci. USA 1989, 86, 9375–9379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callejas, S.; Leech, V.; Reitter, C.; Melville, S. Hemizygous subtelomeres of an African trypanosome chromosome may account for over 75% of chromosome length. Genome Res. 2006, 16, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takle, G.B.; O’Connor, J.; Young, A.J.; Cross, G.A.M. Sequence homology and absence of mRNA defines a possible pseudogene member of the Trypanosoma cruzi gp85/sialidase multigene family. Mol. Biochem. Parasitol. 1992, 56, 117–127. [Google Scholar] [CrossRef]
- Taylor, M.C.; Muhia, D.K.; Baker, D.A.; Mondragon, A.; Schaap, P.; Kelly, J.M. Trypanosoma cruzi adenylyl cyclase is encoded by a complex multigene family. Mol. Biochem. Parasitol. 1999, 104, 205–217. [Google Scholar] [CrossRef]
- Allen, C.L.; Kelly, J.M. Trypanosoma cruzi: Mucin Pseudogenes Organized in a Tandem Array. Exp. Parasitol. 2001, 97, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, G.C.; Bartholomeu, D.C.; DaRocha, W.D.; Hou, L.; Freitas-Silva, D.M.; Machado, C.R.; El-Sayed, N.M.; Teixeira, S.M.R. Sequence diversity and evolution of multigene families in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2008, 157, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.P. Tandem gene arrays in Trypanosoma brucei: Comparative phylogenomic analysis of duplicate sequence variation. BMC Evol. Biol. 2007, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, C.B.; da Cunha, J.P.C.; Inada, D.T.; Damasceno, J.; Lima, A.R.J.; Hiraiwa, P.; Marques, C.; Gonçalves, E.; Nishiyama-Junior, M.Y.; McCulloch, R.; et al. Replication origin location might contribute to genetic variability in Trypanosoma cruzi. BMC Genomics 2020, 21, 414. [Google Scholar] [CrossRef]
- Weir, W.; Capewell, P.; Foth, B.; Clucas, C.; Pountain, A.; Steketee, P.; Veitch, N.; Koffi, M.; de Meeûs, T.; Kaboré, J.; et al. Population genomics reveals the origin and asexual evolution of human infective trypanosomes. Elife 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Parodi-Talice, A.; Durán, R.; Arrambide, N.; Prieto, V.; Piñeyro, M.D.; Pritsch, O.; Cayota, A.; Cerveñansky, C.; Robello, C. Proteome analysis of the causative agent of Chagas disease: Trypanosoma cruzi. Int. J. Parasitol. 2004, 34, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Parodi-Talice, A.; Monteiro-Goes, V.; Arrambide, N.; Avila, A.R.; Duran, R.; Correa, A.; Dallagiovanna, B.; Cayota, A.; Krieger, M.; Goldenberg, S.; et al. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J. Mass Spectrom. 2007, 42, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Brunoro, G.V.F.; Caminha, M.A.; da Silva Ferreira, A.T.; da Veiga Leprevost, F.; Carvalho, P.C.; Perales, J.; Valente, R.H.; Menna-Barreto, R.F.S. Reevaluating the Trypanosoma cruzi proteomic map: The shotgun description of bloodstream trypomastigotes. J. Proteomics 2015, 115, 58–65. [Google Scholar] [CrossRef]
- Bautista-López, N.L.; Ndao, M.; Camargo, F.V.; Nara, T.; Annoura, T.; Hardie, D.B.; Borchers, C.H.; Jardim, A. Characterization and Diagnostic Application of Trypanosoma cruzi Trypomastigote Excreted-Secreted Antigens Shed in Extracellular Vesicles Released from Infected Mammalian Cells. J. Clin. Microbiol. 2017, 55, 744–758. [Google Scholar] [CrossRef] [Green Version]
- Trocoli Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; e Silva, N.C.; de Almeida Abrahamsohn, I.; Colli, W.; Manso Alves, M.J. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; Da Silveira, J.F.; Almeida, I.C. Proteomic analysis of Trypanosoma cruzi secretome: Characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef]
- Brossas, J.Y.; Gulin, J.E.N.; Bisio, M.M.C.; Chapelle, M.; Marinach-Patrice, C.; Bordessoules, M.; Ruiz, G.P.; Vion, J.; Paris, L.; Altcheh, J.; et al. Secretome analysis of Trypanosoma cruzi by proteomics studies. PLoS ONE 2017, 12, e0185504. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Silva, M.R.; Cura Das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; De Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]






| Group | Gene ID TriTrypDB 1 | CDS (bp) 2 | Peptide (aa) 3 | Direction of Transcription 4 | Subtelomeric Region 5 | Chromosome 6 |
|---|---|---|---|---|---|---|
| 1 | TcCLB.511845.10 | 270 | 90 | Sense | - | TcChr20-P (580,762–581,031) |
| TcCLB.509717.176 | 402 | 134 | Sense | - | TcChr4-P (157,230–157,631) | |
| TcCLB.509295.90 | 771 | 256 | Sense | Tel 6 | TcChr28-P (746,714–747,484) | |
| TcCLB.510479.11 | 1701 | 567 | Sense | - | TcChr38-P (1,335,682–1,337,382) | |
| TcCLB.506961.10 | 1929 | 642 | Anti-Sense | - | TcChr18-S (118–2046) | |
| TcCLB.506001.90 | 2763 | 920 | Sense | - | TcChr4-P (166,550–169,312) | |
| TcCLB.507167.70 | 2772 | 923 | Sense | Tel 6 | TcChr28-P (837,994–840,765) | |
| TcCLB.508479.500 | 2892 | 963 | Anti-Sense | - | TcChr40-P (1,914,173–1,917,064) | |
| 2 | TcCLB.509875.11 | 819 | 273 | Sense | Tel 13 | TcChr26-P (793,295–794,113) |
| TcCLB.509873.10 | 831 | 276 | Sense | Tel 13 | TcChr26-P (794,215–795,045) | |
| TcCLB.508285.10 | 1767 | 588 | Sense | Tel 3 | TcChr19-S (653,962–655,728) | |
| TcCLB.506421.10 | 1038 | 345 | Anti-Sense | Tel 49 | TcChr31-P (53,479–54,51) | |
| TcCLB.509915.60 | 1767 | 588 | Anti-Sense | Tel 49 | TcChr31-P (64,469–66,235) | |
| TcCLB.506443.150 | 2400 | 799 | Sense | Tel 24 | TcChr11-P (510,464–512,863) | |
| TcCLB.507555.80 | 2757 | 918 | Anti-Sense | Tel 35 | TcChr35-S (510,464–512,863) | |
| 3 | TcCLB.459199.10 | 2820 | 939 | Anti-Sense | Tel 28 | TcChr15-P (5578–8397) |
| TcCLB.506047.20 | 1815 | 604 | Sense | Tel 9 | TcChr35-S (1,183,688–1,185,502) | |
| TcCLB.506017.51 | 1122 | 374 | Sense | - | TcChr29-P (869,711–870,832) | |
| TcCLB.507167.20 | 2835 | 944 | Sense | Tel 6 | TcChr28-P (849,015–851,849) | |
| TcCLB.507611.10 | 2841 | 946 | Anti-Sense | Tel 17 | TcChr37-S (1391–4231) | |
| TcCLB.506393189 | 2274 | 758 | Sense | - | TcChr14-P (596,251–598,524) | |
| TcCLB.506323.30 | 2790 | 929 | Anti-Sense | Tel 4 | TcChr22-P (62,292–65,081) | |
| TcCLB.509429.4 | 2613 | 871 | Sense | - | TcChr6-P (364,778–367,390) | |
| TcCLB.511773.110 | 2472 | 995 | Anti-Sense | - | TcChr17-P (301–2772) | |
| TcCLB.508037.10 | 1146 | 381 | Anti-Sense | Tel 48 | TcChr27-S (1297–2442) | |
| TcCLB.511929.30 | 2781 | 926 | Sense | - | TcChr25-P (736,933–739,713) | |
| TcCLB.504109.200 | 3294 | 1097 | Anti-Sense | - | TcChr39-P (599–3892) | |
| TcCLB.508473.10 | 4512 | 1503 | Sense | Tel 30 | TcChr39-S (1,847,980–1,852,491) | |
| TcCLB.507625.10 | 4149 | 1382 | Sense | Tel 45 | TcChr40-S (1,133,828–1,137,976) | |
| TcCLB.39997.10 | 1053 | 350 | Anti-Sense | - | TcChr37-P (33,320–34,372) | |
| 4 | TcCLB.504343.30 | 1779 | 592 | Anti-Sense | - | TcChr7-S (60,071–61,849) |
| TcCLB.507.907.30 | 1779 | 592 | Anti-Sense | - | TcChr7-S (73,533–75,311) | |
| TcCLB.507.907.60 | 1779 | 592 | Anti-Sense | - | TcChr7-S (62,859–64,637) | |
| TcCLB.505207.30 | 1626 | 541 | Anti-Sense | - | TcChr41-P (8244–9869) | |
| 5 | TcCLB.511019.80 * | 1500 | 499 | Sense | - | TcChr35-P (101,616–103,187) |
| TcCLB.503881.30 | 1509 | 502 | Sense | - | TcChr33-S (730,729–732,237) | |
| TcCLB.508119.140 | 1503 | 500 | Anti-Sense | - | TcChr33-P (724,554–726,056) | |
| TcCLB.511907.330 | 1503 | 500 | Sense | - | TcChr26-P (250,686–252,188) | |
| TcCLB.506529.680 | 444 | 148 | Sense | - | TcChr6-S (201,683–202,126 | |
| TcCLB.510889.352 | 510 | 170 | Sense | - | TcChr6-P (201,577–202,086 | |
| 6 | TcCLB.509085.120 | 1896 | 631 | Anti-Sense | - | TcChr15-P (164,566–166,461 |
| TcCLB.509437.110 | 1896 | 631 | Sense | - | TcChr15-P (256,827–258,722 | |
| TcCLB.509349.20 | 1893 | 630 | Anti-Sense | Tel 2 | TcChr11-S (115,973–117,865) | |
| TcCLB.508479.80 | 1947 | 648 | Sense | - | TcChr40-P (1,993,454–1,995,400) | |
| TcCLB.509163.110 | 1962 | 653 | Sense | - | TcChr35-P (1,138,639–1,140,600) | |
| TcCLB.511871.130 | 1896 | 631 | Sense | - | TcChr15-S (101,636–103,531) | |
| TcCLB.511861.90 | 1896 | 631 | Sense | - | TcChr15-P (118,605–120,500) | |
| TcCLB.511863.4 | 1572 | 524 | Sense | - | TcChr15-P (101,616–103,187) | |
| 7 | TcCLB.506809.5 | 354 | 117 | Sense | - | TcChr16-P (453,466–453,819) |
| TcCLB.509575.10 | 2763 | 920 | Sense | - | TcChr16-P (389,424–392,186) | |
| TcCLB.424771.10 | 873 | 290 | Sense | - | TcChr16-P (477,440–478,312) | |
| TcCLB.507843.10 | 1779 | 592 | Sense | - | TcChr16-S (390,540–392,318) | |
| TcCLB.509827.4 | 962 | 320 | Sense | - | TcChr16-S (389,477–390,438) | |
| TcCLB.507841.14 | 2562 | 854 | Sense | - | TcChr16-S (452,820–455,381) | |
| 8 | TcCLB.511019.13 | 1548 | 516 | Sense | - | TcChr35-P (446,350–447,897) |
| TcCLB.509219.20 | 3633 | 1210 | Sense | - | TcChr20-P (567,813–571,445) | |
| TcCLB.506271.30 | 324 | 108 | Sense | - | TcChr20-P (586,959–587,282) | |
| TcCLB.510643.190 | 2496 | 831 | Sense | - | TcChr16-P (642,806–645,301) | |
| TcCLB.505997.60 | 2316 | 771 | Anti-Sense | Tel 1 | TcChr9-P (12,280–14,595) | |
| TcCLB.506595.149 | 2465 | 821 | Anti-Sense | - | TcChr33-P (101–2565) | |
| TcCLB.511371.10 | 1785 | 594 | Sense | - | TcChr5-S (200,095–201,879) | |
| TcCLB.511415.11 | 1095 | 365 | Anti-Sense | - | TcChr9-S (30,121–31,215) | |
| TcCLB.508559.90 | 1821 | 606 | Sense | Tel 21 | TcChr25-S (700,188–702,008) | |
| TcCLB.511585.320 | 1932 | 643 | Anti-Sense | Tel 14 | TcChr33-S (31,331–33,262) | |
| TcCLB.507015.10 * | 2988 | 995 | Anti-Sense | Tel 10 | TcChr13-P (1626–4613) | |
| TcCLB.509917.19 | 1815 | 605 | Anti-Sense | Tel 49 | TcChr31-P (54,619–56,433) | |
| 9 | TcCLB.503401.11 | 243 | 81 | Sense | - | TcChr22-S (214,572–214,814) |
| TcCLB.506629.240 | 327 | 109 | Anti-Sense | - | TcChr39-P (389,442–389,768) | |
| TcCLB.509829.9 | 909 | 303 | Anti-Sense | - | TcChr39-S 392,244–393) | |
| TcCLB.509329.9 | 752 | 250 | Sense | - | TcChr22-P (339,264–340,015) | |
| TcCLB.509463.41 * | 1209 | 403 | Anti-Sense | - | TcChr22-P (391,811–393,019) | |
| TcCLB.509843.10 | 1503 | 500 | Sense | - | TcChr22-S (214,918–216,420) | |
| 10 | TcCLB.506139.200 | 1674 | 557 | Sense | - | TcChr18-P (357,746–359,419) |
| TcCLB.510845.10 | 1824 | 608 | Anti-Sense | - | TcChr19-S (28,739–30,562) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, W.P.; Souza, R.T.; Costa-Martins, A.G.; Ferreira, E.R.; Mortara, R.A.; Teixeira, M.M.G.; Ramirez, J.L.; Da Silveira, J.F. Genomic Organization and Generation of Genetic Variability in the RHS (Retrotransposon Hot Spot) Protein Multigene Family in Trypanosoma cruzi. Genes 2020, 11, 1085. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11091085
Bernardo WP, Souza RT, Costa-Martins AG, Ferreira ER, Mortara RA, Teixeira MMG, Ramirez JL, Da Silveira JF. Genomic Organization and Generation of Genetic Variability in the RHS (Retrotransposon Hot Spot) Protein Multigene Family in Trypanosoma cruzi. Genes. 2020; 11(9):1085. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11091085
Chicago/Turabian StyleBernardo, Werica P., Renata T. Souza, André G. Costa-Martins, Eden R. Ferreira, Renato A. Mortara, Marta M. G. Teixeira, José Luis Ramirez, and José F. Da Silveira. 2020. "Genomic Organization and Generation of Genetic Variability in the RHS (Retrotransposon Hot Spot) Protein Multigene Family in Trypanosoma cruzi" Genes 11, no. 9: 1085. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11091085