Investigating the Adoption of Clinical Genomics in Australia. An Implementation Science Case Study
Abstract
:1. Introduction
2. Materials and Methods
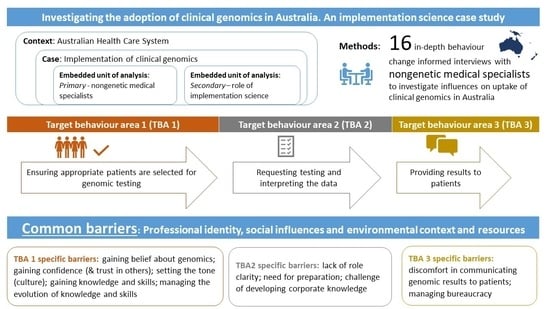
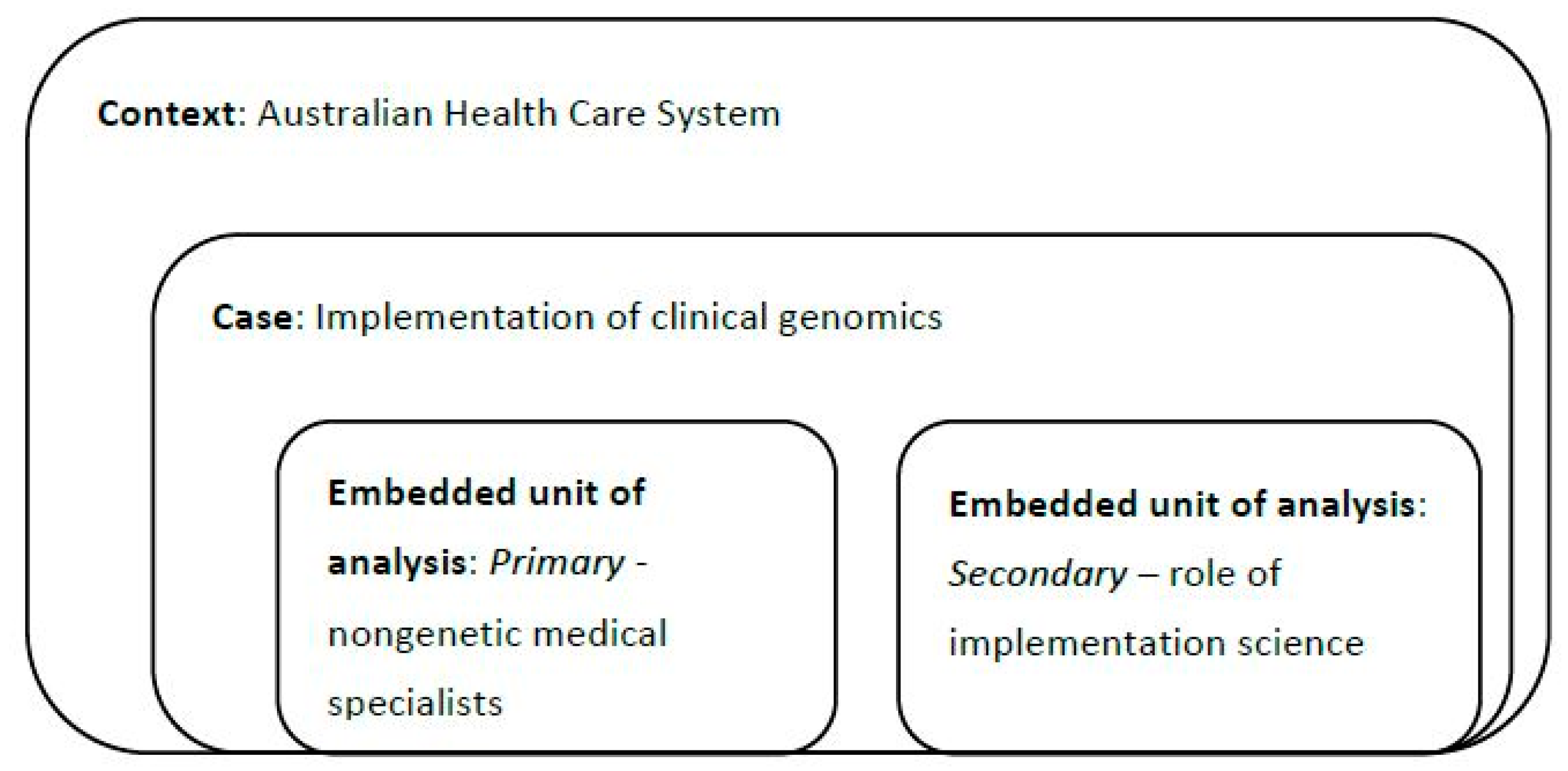
2.1. Context
2.2. Research Design
2.3. Participants and Recruitment
2.4. Data Collection, Procedure and Analysis
2.4.1. Interview Development
2.4.2. Conducting the Interview
2.4.3. Data Analysis
3. Results
3.1. Demographics
3.2. Target Behaviour Areas 1–3
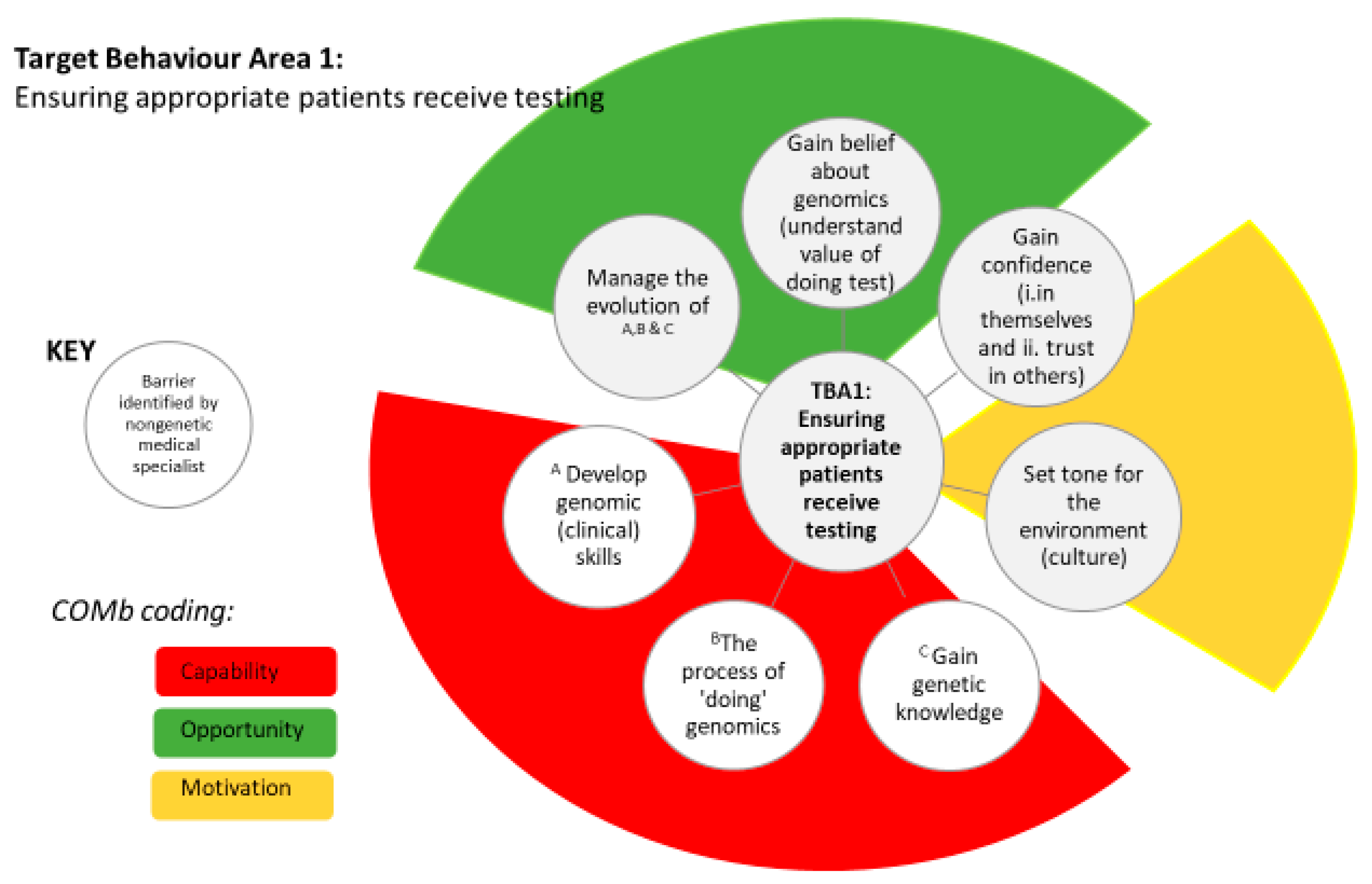
3.3. Target Behaviour Area 1
3.4. Target Behaviour Area 2
3.5. Target Behaviour Area 3
4. Discussion
4.1. Primary Embedded Unit of Analysis: Nongenetic Medical Specialists
4.2. Secondary Embedded Unit of Analysis: Role of Implementation Science
4.3. Theory-Based Targeted Interventions to Support the Implementation of Clinical Genomics
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stark, Z.; Dolman, L.; Manolio, T.A.; Ozenberger, B.; Hill, S.L.; Caulfied, M.J.; Levy, Y.; Glazer, D.; Wilson, J.; Lawler, M.; et al. Integrating Genomics into Healthcare: A Global Responsibility. Am. J. Hum. Genet. 2019, 104, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Government, Medicare Benefits Schedule MBS Online. Available online: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=73358&qt=item (accessed on 23 February 2021).
- Johnson, K.B.; Clayton, E.W.; Starren, J.; Peterson, J. The Implementation Chasm Hindering Genome-informed Health Care. J. Law Med. Ethic 2020, 48, 119–125. [Google Scholar] [CrossRef]
- Birken, S.A.; Bunger, A.C.; Powell, B.J.; Turner, K.; Clary, A.S.; Klaman, S.L.; Yu, Y.; Whitaker, D.J.; Self, S.R.; Rostad, W.L.; et al. Organizational theory for dissemination and implementation research. Implement. Sci. 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, F.; Dixon-Woods, M.; Leviton, L.; Michie, S. Demystifying theory and its use in improvement. BMJ Qual. Saf. 2015, 24, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Lynch, E.A.; Mudge, A.; Knowles, S.; Kitson, A.L.; Hunter, S.C.; Harvey, G. “There is nothing so practical as a good theory”: A pragmatic guide for selecting theoretical approaches for implementation projects. BMC Health Serv. Res. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Sperber, N.R.; Carpenter, J.S.; Cavallari, L.H.; Damschroder, L.J.; Cooper-DeHoff, R.M.; Denny, J.C.; Ginsburg, G.S.; Guan, Y.; Horowitz, C.R.; Levy, K.D.; et al. Challenges and strategies for implementing genomic services in diverse settings: Experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med. Genom. 2017, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, K.; Quinlan, C.; Mallett, A.J.; Kerr, P.G.; McClaren, B.; Nisselle, A.; Mallawaarachchi, A.; Polkinghorne, K.R.; Patel, C.; Best, S.; et al. Attitudes and Practices of Australian Nephrologists Toward Implementation of Clinical Genomics. Kidney Int. Rep. 2020. [Google Scholar] [CrossRef]
- Rifkin, W.; Fulop, L. A review and case study on learning organizations. Learn. Organ. 1997, 4, 135–148. [Google Scholar] [CrossRef]
- Matlay, H. Industry—higher education collaborations within small business clusters: Evidence from UK case studies. Ind. Higher Educ. 2000, 12, 386–393. [Google Scholar] [CrossRef]
- Baxter, P.; Jack, S. Qualitative Case Study Methodology: Study Design and Implementation for Novice Researchers. TQR 2008, 13, 544–559. [Google Scholar]
- Flyvbjerg, B. Case Study. In The Sage Handbook of Qualitative Research, 4th ed.; Denzin, N.K., Lincoln, Y.S., Eds.; Sage: Thousand Oaks, CA, USA, 2011; pp. 301–316. [Google Scholar]
- Kohn, L.T. Methods in Case Study Analysis; Technical Report for the Center for Studying Health System Change; Center for Studying Health System Change: Washington, DC, USA, 1997. [Google Scholar]
- Cooper, H.; Camic, P.M.; Long, D.L.; Panter, A.T.; Rindskopf, D.; Sher, K.J. Research designs: Quantitative, qualitative, neuropsychological, and biological. In APA Handbooks in Psychology® APA Handbook of Research Methods in Psychology; American Psychological Association: Washington, DC, USA, 2012; Volume 2, pp. 141–155. [Google Scholar]
- Stark, Z.; Boughtwood, T.; Phillips, P.; Christodoulou, J.; Hansen, D.P.; Braithwaite, J.; Newson, A.J.; Gaff, C.L.; Sinclair, A.H.; North, K.N. Australian Genomics: A Federated Model for Integrating Genomics into Healthcare. Am. J. Hum. Genet. 2019, 105, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaff, C.L.; Winship, I.M.; Forrest, S.M.; Hansen, D.P.; Clark, J.; Waring, P.M.; South, M.; Sinclair, A.H. Preparing for genomic medicine: A real world demonstration of health system change. NPJ Genom. Med. 2017, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation Hybrid Designs. Med. Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, N.; Best, S.; Martyn, M.; Long, J.C.; North, K.N.; Braithwaite, J.; Gaff, C. A transformative translational change programme to introduce genomics into healthcare: A complexity and implementation science study protocol. BMJ Open 2018, 9, e024681. [Google Scholar] [CrossRef] [Green Version]
- Dyson, J.; Lawton, R.; Jackson, C.; Cheater, F. Does the use of a theoretical approach tell us more about hand hygiene behaviour? The barriers and levers to hand hygiene. J. Infect. Prev. 2010, 12, 17–24. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Francis, J.; Hardeman, W.; Eccles, M. From Theory to Intervention: Mapping Theoretically Derived Be-havioural Determinants to Behaviour Change Techniques. Appl. Psychol. 2008, 57, 660–680. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Crellin, E.; McClaren, B.; Nisselle, A.; Best, S.; Gaff, C.; Metcalfe, S. Preparing Medical Specialists to Practice Genomic Medicine: Education an Essential Part of a Broader Strategy. Front. Genet. 2019, 10, 789. [Google Scholar] [CrossRef]
- Michaelson-Cohen, R.; Salzer-Sheelo, L.; Sukenik-Halevy, R.; Koifman, A.; Fellner, A.; Reches, A.; Marom, D.; Behar, D.M.; Sofrin-Drucker, E.; Zaks-Hoffer, G.; et al. Teaching clinicians practical genomic medicine: 7 years’ experience in a tertiary care center. Genet. Med. 2020, 22, 1703–1709. [Google Scholar] [CrossRef]
- Newson, A.J.; Leonard, S.J.; Hall, A.; Gaff, C.L. Known unknowns: Building an ethics of uncertainty into genomic medicine. BMC Med. Genom. 2016, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.; Carey, R.N.; Bohlen, L.E.C.; Johnston, D.W.; Rothman, A.J.; De Bruin, M.; Kelly, M.P.; Groarke, H.; Michie, S. Development of an online tool for linking behavior change techniques and mechanisms of action based on triangulation of findings from literature synthesis and expert consensus). Transl. Behav. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Waltz, T.J.; Powell, B.J.; Fernández, M.E.; Abadie, B.; Damschroder, L.J. Choosing implementation strategies to address contextual barriers: Diversity in recommendations and future directions. Implement. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm. Policy Ment. Health Ment. Health Serv. Res. 2010, 38, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, P. Making sense of implementation theories, models and frameworks. Implement. Sci. 2015, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.L.; Leslie, H.; Trainer, A.H.; Gaff, C. A theory-informed systematic review of clinicians’ genetic testing practices. Eur. J. Hum. Genet. 2018, 26, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Lawton, R.; Programme, O.B.O.T.A.; Heyhoe, J.; Louch, G.; Ingleson, E.; Glidewell, L.; Willis, T.A.; McEachan, R.R.C.; Foy, R. Using the Theoretical Domains Framework (TDF) to understand adherence to multiple evidence-based indicators in primary care: A qualitative study. Implement. Sci. 2015, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Foy, R.; Ovretveit, J.; Shekelle, P.G.; Pronovost, P.J.; Taylor, S.L.; Dy, S.; Hempel, S.; McDonald, K.M.; Rubenstein, L.V.; Wachter, R.M. The role of theory in research to develop and evaluate the implementation of patient safety practices. BMJ Qual. Saf. 2011, 20, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Presseau, J.; Mutsaers, B.; Al-Jaishi, A.A.; Squires, J.; McIntyre, C.W.; Garg, A.X.; Sood, M.M.; Grimshaw, J.M. Barriers and facilitators to healthcare professional behaviour change in clinical trials using the Theoretical Domains Framework: A case study of a trial of individualized temperature-reduced haemodialysis. Trials 2017, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sargent, L.; McCullough, A.; Del Mar, C.; Lowe, J. Using theory to explore facilitators and barriers to delayed prescribing in Aus-tralia: A qualitative study using the Theoretical Domains Framework and the Behaviour Change Wheel. BMC FAM Pract. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Taylor, N.; Healey, E.; Morrow, A.; Greening, S.; Wakefield, C.E.; Warwick, L.; Williams, R.; Tucker, K.M. Aligning intuition and theory: Enhancing the replicability of behaviour change interventions in cancer genetics. Implement. Sci. Commun. 2020, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Glasziou, P.; Altman, D.G.; Bossuyt, P.; Boutron, I.; Clarke, M.; Julious, S.; Michie, S.; Moher, D.; Wager, E. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014, 383, 267–276. [Google Scholar] [CrossRef]
- Michie, S.; Atkins, L.; West, R. The Behaviour Change Wheel, A Guide to Designing Interventions; Silverback Publishing: Sutton, UK, 2014. [Google Scholar]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Clinical Speciality | No. of Participants |
|---|---|
| Neurology | 4 |
| Cardiology | 1 |
| Nephrology | 6 |
| Immunology | 2 |
| Oncology | 3 |
| Subtotal | 16 |
| COM | Barriers | Example Quote (Related to the Bolded Barrier) | Enablers |
|---|---|---|---|
| C | Gain belief about genomics e.g., understanding the value of doing the test (TDF domain: belief about consequences) | ||
| There is a lack of natural referral patterns, and time requirements, which make it more challenging to appreciate the value of testing as clinicians are time poor | We have busy clinics here. This, you know, one of the things that (this genomics project is) supposed to be done in the context of a routine clinic. No chance. No chance at all. NGMS11 | Seeing results NGMS 1, 2, 6 | |
| Gain confidence in (i) themselves (ii) trust in others e.g., to gain/grow i.e., genetic knowledge and skills (TDF domain: i) belief about capabilities and ii) social influences) | |||
| O | Challenging to gain/grow genetic knowledge and skills because they are unable to join meetings to grow confidence, not trained to counsel, lack of experience, lack of GC at offering stage to build confidence | I learnt a huge amount from our genetic counsellors on how they consent. I don’t think, to start off with, I would have appreciated that that was important and I think, because my experience would be fairly reflective of most (physicians), nobody told me that I needed to worry so much about incidental findings ‘cause nobody—no other (physicians) appreciated that so I didn’t know that so I didn’t do that or I wouldn’t have done it. NGMS4 | Having knowledgeable person to aske informal questions NGMS2 CGs and GCs to support NGMS3 Lab experience NGMS5 Gaining experience NGMS9 Informal discussions with colleagues NGMS15 Research programme experience NGMS1 Easy to explain to patients in research setting NGMS13 Access to other physicians with genetic knowledge NGMS12 |
| Set the tone for environment (culture) e.g., running meetings (TDF domain: social influences) | |||
| How meetings are run | No barriers noted—enabler coded | Congenial relationships NGMS16 Personal contact with physicians NGMS3 Small local meetings best for info sharing NGMS4 | |
| M | A Gain genetic knowledge e.g., Who to refer? What conditions might have a genetic basis? (TDF domain: knowledge) | ||
| Need to know who to refer and what conditions might have a genetic basis. There is a gap in understanding of who to select, lack of awareness of how many conditions may be genetic and a need for people to understand the whole process not just the test | The issue is that people (nongenetic medical specialists) really need to change thinking because people (nongenetic medical specialists) don’t think about this being genetic. NGMS7 | Dynamic and fluid checklists for selecting patients NGMS13 Interim gatekeeping as knowledge grows NGMS13 Training NGSM12 | |
| B Find out about the process of “doing” genomics e.g., information about consent processes, how to access services (TDF domain: skills) | |||
| Lack of information about consent processes with no (formal) consent training and how to access services with no centralised/established path | I think historically we haven’t been particularly well trained as adult physicians (about consent). NGMS12 | Clear referral criteria Having an informal checklist (clinical reasoning) NGMS10-links to confidence | |
| C Develop genomic (clinical) skills e.g., getting hands-on (TDF domain: skills) | |||
| Getting hands-on with the process of clinical genomics | I think the evolution of clinical expertise and practice over years (helps gain clinical skills). Did I go to any training course to talk about it or anything like that? No. It’s just something that you, another bit of information and a skill that you acquire. NGMS10 | Seeking feedback, reflecting NGMS4 Gained during the research process NGMS7 Scientists “dumbing down” information NGMS8—ties to gain confidence | |
| C | Managing the evolution of motivation barriers A,B,C e.g., knowledge shifting, processes changing (TDF domain: professional identity and belief about capabilities) | ||
| Knowledge is shifting and the evidence base is dynamic and fluid, so processes are changing. The lack of guidance leads to relying on own clinical experience, and hindered by a lack of genomic literacy | Even if we were to limit our interest to (one clinical area) genetics, it’s still a very dynamic and fluid field. Even the most enthusiastic have a hard time to keep up with the research discoveries—which happen, almost on a monthly basis, you have a new gene associated with a condition. NGMS1 | “Generational shift” younger practitioners will come in with knowledge NGMS5 Summaries of latest evidence NGMS3 | |
| COM | Barriers | Example Quote (Related to the Bolded Barrier) | Enablers |
|---|---|---|---|
| C | Role clarity e.g., different craft groups undertake different roles (TDF domain: professional identity) | ||
| Lack of clinician genetic literacy Risk of over-enthusiastic/lack of confidence in call variants | I didn’t really understand a lot of this—the genomics science stuff and then the scientist didn’t really understand because we were talking about the clinical phenotypes—which, I think, is actually really important. NGMS2 | Understanding clinician and scientist sides NGMS2 Allowing evolution of roles as comfort grows NGMS14 Meetings to break down barriers NGMS8 | |
| O | Need for preparation e.g., practitioners need to research and plan in order to participate (TDF domain: environmental context and resources) | ||
| Lack of time and effort into learning new approach | What is challenging is the variant interpretation and that’s where, I think, your average (medical specialist) is not going to have the time or interest to invest. NGMS16 | Everyone is engaged and able to speak openly NGMS4 | |
| Developing corporate knowledge: the idea that everyone is collectively developing their knowledge, commonly through MDTs and through other avenues | |||
| O | Developing corporate knowledge: building relationships (TDF domain: social influences) | ||
| Not understanding each other’s roles No meetings | I think, it would be nice to have a particular person or go-to person, that you have a good relationship with that you can just, kind of, go bounce off questions or ideas without having to even sometimes formally… maybe, a different genetic counsellor and different, it’s just a little bit hard to know where to go. NGMS2 | Communication NGMS1 Regular constructive meetings NGMS8 | |
| M | Developing corporate knowledge: trust between professionals (TDF domain: emotion) | ||
| Less trust when there are no MDTs Trust in labs | “Well what’s the turnaround time?”, “Do they trust the results from that lab?” NGMS12 | Important to develop trusting relationships with genetics NGMS9 Meetings to discuss patients NGMS8 Good clinical trust permits discussion NGMS5 | |
| Developing corporate knowledge: safety in being vulnerable (TDF domain: emotion) | |||
| Embarrassment at not understanding the process. Lack of comfort calling variants alone | All that kind of the jargon about how relevant a variant might be, I found that was just all a bit of gobbledygook, and I, kind of, had to stop and ask, you know, at first I was a bit embarrassed because I thought everyone else knew and then I realised that the person next to me who was a clinician also had no idea what they were talking about, so it was just, kind of, yeah, and it was just good to acknowledge at the beginning that these are two different languages and how we’re going to meet in the middle. NGMS2 | Getting involved NGMS12 Access to support when needed NGMS2 Positive open culture NGMS4 | |
| Developing corporate knowledge: still learning (TDF domain: intentions) | |||
| We’re still learning Still need support of other professionals | I’m still very much learning? Very much learning about what it all means and very much guided by—we’re very lucky here ‘cause we do have the geneticists and things, so they can help talk a little—“well this is what it means”. So I’m learning. NGMS12 | Discussion promotes “automatic” learning, NGMS9 | |
| COM | Barriers | Example Quote (Related to the Bolded Barrier) | Enablers |
|---|---|---|---|
| O | Managing bureaucracy (TDF domain: environment and resources) | ||
| Bureaucracy Vague reports Speed of results | (Challenges from the delay in getting results) Often we’ve forgotten that we’ve tested (the patient) until next time they come and goes, “Oh, yes, what happened to that genetic testing”. They (NGMS) go, “Oh, look yes, we do have a result. Um, it’s going to be too hard for me to, interpret it for you now, give me a day and I’ll talk to people and then get back to you”, type of scenario. NGMS15 | Clear lab reports NGMS15 Clarity in letters to referrers NGMS4 | |
| M | Comfort in communicating genomic results to patients (TDF domain: professional identity and social influences) | ||
| Evolving field Working in isolation | Things evolve and so—and the art in what we do or the challenge in what we do is deciding at which stage to intervene. NGMS10 | Professional confidence (“it’s a call I make”) NGMS6 Access to experts NGMS3 Proximity to experts NGMS9 Relationships with genetic professionals NGMS12 Genomics experience NGMS5 Preparation NGMS4 | |
| Barrier | TDF Code | TDF Theory Informed Behaviour Change Technique | Applied in Context |
|---|---|---|---|
| The need to gain belief about genomics, and an appreciation of the value of doing genomic testing | Belief about consequences | e.g., “salience of consequences” | e.g., providing evidence of the impact of genomics, for example through seeing a patient receive a diagnosis from genomic testing |
| The need to gain confidence in themselves and trust in others | Belief about capabilities | e.g., “demonstration of the behaviour” | e.g., providing an observable example, for example with a video or observing others using clinical genomics in clinic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Best, S.; Long, J.C.; Gaff, C.; Braithwaite, J.; Taylor, N. Investigating the Adoption of Clinical Genomics in Australia. An Implementation Science Case Study. Genes 2021, 12, 317. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12020317
Best S, Long JC, Gaff C, Braithwaite J, Taylor N. Investigating the Adoption of Clinical Genomics in Australia. An Implementation Science Case Study. Genes. 2021; 12(2):317. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12020317
Chicago/Turabian StyleBest, Stephanie, Janet C. Long, Clara Gaff, Jeffrey Braithwaite, and Natalie Taylor. 2021. "Investigating the Adoption of Clinical Genomics in Australia. An Implementation Science Case Study" Genes 12, no. 2: 317. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12020317