SLC26A2-Associated Diastrophic Dysplasia and rMED—Clinical Features in Affected Finnish Children and Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
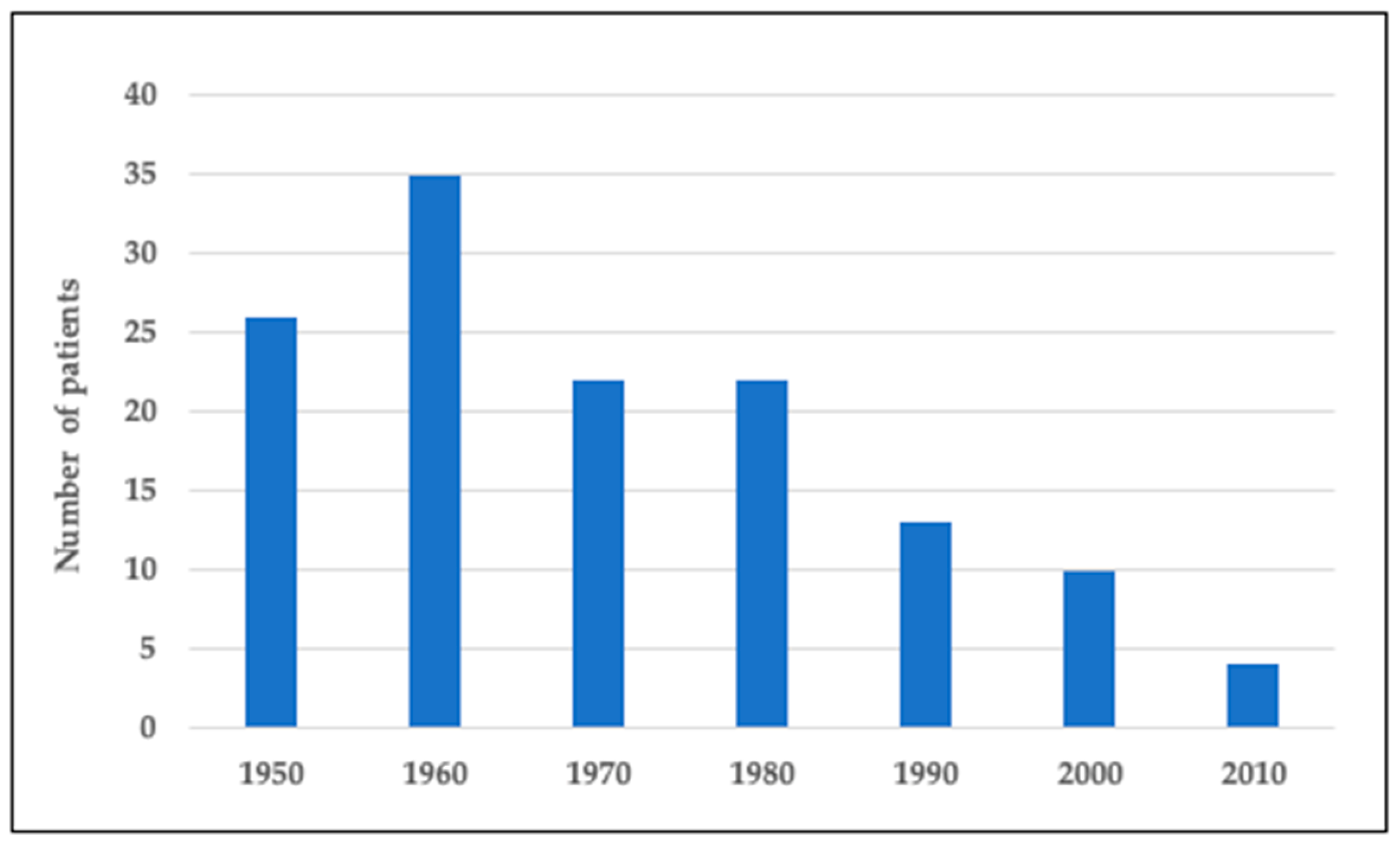
3.1. Number of Finnish DTD Subjects Born in 1950–2020
3.2. Clinical and Molecular Description of the Pediatric Cohort
3.3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hästbacka, J.; De La Chapelle, A.; Mahtani, M.M.; Clines, G.; Reeve-Daly, M.P.; Daly, M.; Hamilton, B.A.; Kusumi, K.; Trivedi, B.; Weaver, A.; et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: Positional cloning by fine-structure linkage disequilibrium mapping. Cell 1994, 78, 1073–1087. [Google Scholar] [CrossRef]
- Forlino, A.; Piazza, R.; Tiveron, C.; Della Torre, S.; Tatangelo, L.; Bonafè, L.; Gualeni, B.; Romano, A.; Pecora, F.; Superti-Furga, A.; et al. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: Morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum. Mol. Genet. 2005, 14, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Haila, S.; Hästbacka, J.; Böhling, T.; Karjalainen-Lindsberg, M.-L.; Kere, J.; Saarialho-Kere, U. SLC26A2 (Diastrophic Dysplasia Sulfate Transporter) is Expressed in Developing and Mature Cartilage but Also in Other Tissues and Cell Types. J. Histochem. Cytochem. 2001, 49, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Ohana, E.; Choi, S.Y.; Lee, M.-S.; Park, J.H.; Muallem, S. Multiple Roles of the SO42−/Cl−/OH− Exchanger Protein Slc26a2 in Chondrocyte Functions. J. Biol. Chem. 2014, 289, 1993–2001. [Google Scholar] [CrossRef] [Green Version]
- Superti-Furga, A.; Rossi, A.; Steinmann, B.; Gitzelmann, R. A chondrodysplasia family produced by mutations in the diastrophic dysplasia sulfate transporter gene: Genotype/phenotype correlations. Am. J. Med. Genet. 1996, 63, 144–147. [Google Scholar] [CrossRef]
- Rossi, A.; Kaitila, I.; Wilcox, W.R.; Rimoin, D.L.; Steinmann, B.; Cetta, G.; Superti-Furga, A. Proteoglycan sulfation in cartilage and cell cultures from patients with sulfate transporter chondrodysplasias: Relationship to clinical severity and indications on the role of intracellular sulfate production. Matrix Biol. 1998, 17, 361–369. [Google Scholar] [CrossRef]
- Mortier, G.R.; Cohn, D.H.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Mundlos, S.; Nishimura, G.; Robertson, S.; Sangiorgi, L.; Savarirayan, R.; et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet. Part A 2019, 179, 2393–2419. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Superti-Furga, A. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum. Mutat. 2001, 17, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bonafé, L.; Mittaz-Crettol, L.; Ballhausen, D.; Superti-Furga, A. Diastrophic Dysplasia. In GeneReviews(®®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; Copyright © 2021–2020, GeneReviews Is a Registered Trademark of the University of Washington, All Rights Reserved; University of Washington: Seattle, WA, USA, 2013; p. 11. [Google Scholar]
- Hastbacka, J.; Kaitila, I.; Sistonen, P.; de la Chapelle, A. Diastrophic dysplasia gene maps to the distal long arm of chromosome 5. Proc. Natl. Acad. Sci. USA 1990, 87, 8056–8059. [Google Scholar] [CrossRef] [Green Version]
- Norio, R. The Finnish disease heritage III: The individual diseases. Qual. Life Res. 2003, 112, 470–526. [Google Scholar] [CrossRef] [PubMed]
- Hästbacka, J.; Kerrebrock, A.; Mokkala, K.; Clines, G.; Lovett, M.; Kaitila, I.; De La Chapelle, A.; Lander, E.S. Identification of the Finnish founder mutation for diastrophic dysplasia (DTD). Eur. J. Hum. Genet. 1999, 7, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Sousa, A.B.; Medeira, A.; Lourenço, T.; Saraiva, J.; Pinto-Basto, J.; Soares, G.; Fortuna, A.; Superti-Furga, A.; Mittaz, L.; et al. Clinical and molecular characterization of Diastrophic Dysplasia in the Portuguese population. Clin. Genet. 2010, 80, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, O.; Kaitila, I. Growth in diastrophic dysplasia. J. Pediatr. 1997, 130, 641–646. [Google Scholar] [CrossRef]
- Remes, V.; Poussa, M.; Lönnqvist, T.; Puusa, A.; Tervahartiala, P.; Helenius, I.; Peltonen, J. Walking Ability in Patients with Diastrophic Dysplasia: A Clinical, Electroneurophysiological, Treadmill, and MRI Analysis. J. Pediatr. Orthop. 2004, 24, 546–551. [Google Scholar] [CrossRef]
- Krüger, L.; Pohjolainen, T.; Kaitila, I.; Kautiainen, H.; Arkela-Kautiainen, M.; Hurri, H. Health-related quality of life and socioeconomic situation among diastrophic dysplasia patients in Finland. J. Rehabil. Med. 2013, 45, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballhausen, D.; Bonafé, L.; Terhal, P.; Unger, S.L.; Bellus, G.; Classen, M.; Hamel, B.C.; Spranger, J.; Zabel, B.; Cohn, D.H.; et al. Recessive multiple epiphyseal dysplasia (rMED): Phenotype delineation in eighteen homozygotes for DTDST mutation R279W. J. Med. Genet. 2003, 40, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Mäkitie, O.; Savarirayan, R.; Bonafé, L.; Robertson, S.; Susic, M.; Superti-Furga, A.; Cole, W.G. Autosomal recessive multiple epiphyseal dysplasia with homozygosity for C653S in theDTDSTgene: Double-layer patella as a reliable sign. Am. J. Med. Genet. Part A 2003, 122A, 187–192. [Google Scholar] [CrossRef]
- Unger, S.; Bonafé, L.; Superti-Furga, A. Multiple epiphyseal dysplasia: Clinical and radiographic features, differential diagnosis and molecular basis. Best Pr. Res. Clin. Rheumatol. 2008, 22, 19–32. [Google Scholar] [CrossRef]
- Superti-Furga, A.; Neumann, L.; Riebel, T.; Eich, G.; Steinmann, B.; Spranger, J.; Kunze, J. Recessively inherited multiple epiphyseal dysplasia with normal stature, club foot, and double layered patella caused by a DTDST mutation. J. Med. Genet. 1999, 36, 621–624. [Google Scholar]
- Honório, J.C.; Bruns, R.F.; Gründtner, L.F.; Raskin, S.; Ferrari, L.P.; Júnior, E.A.; Nardozza, L.M.M. Diastrophic dysplasia: Prenatal diagnosis and review of the literature. Sao Paulo Med. J. 2013, 131, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Al Kaissi, A.; Kenis, V.; Melchenko, E.; Ben Chehida, F.; Ganger, R.; Klaushofer, K.; Grill, F. Corrections of Lower Limb Deformities in Patients with Diastrophic Dysplasia. Orthop. Surg. 2014, 6, 274–279. [Google Scholar] [CrossRef]
- Helenius, I.; Remes, V.; Lohman, M.; Tallroth, K.; Poussa, M.; Helenius, M.; Paavilainen, T. Total knee arthroplasty in patients with diastrophic dysplasia. JBJS 2003, 85, 2097–2102. [Google Scholar] [CrossRef]
- Helenius, I.; Remes, V.; Tallroth, K.; Peltonen, J.; Poussa, M.; Paavilainen, T. Total hip arthroplasty in diastrophic dysplasia. JBJS 2003, 85, 441–447. [Google Scholar] [CrossRef]
- Jalanko, T.; Remes, V.; Peltonen, J.; Poussa, M.; Helenius, I. Treatment of spinal deformities in patients with diastrophic dysplasia. Spine 2009, 34, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.; Paganini, C.; Lecci, S.; De Leonardis, F.; Villani, S.; Forlino, A.; Hay, E.; Cohen-Solal, M.; Superti-Furga, A.; Tenni, R.; et al. N-acetylcysteine treatment ameliorates the skeletal phenotype of a mouse model of diastrophic dysplasia. Hum. Mol. Genet. 2015, 24, 5570–5580. [Google Scholar] [CrossRef] [Green Version]
- Paganini, C.; Tota, C.G.; Monti, L.; Monti, I.; Maurizi, A.; Capulli, M.; Bourmaud, M.; Teti, A.; Cohen-Solal, M.; Villani, S.; et al. Improvement of the skeletal phenotype in a mouse model of diastrophic dysplasia after postnatal treatment with N-acetylcysteine. Biochem. Pharmacol. 2021, 185, 114452. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Lin, X.; Xu, X.; Wang, C.; Zhou, J.; Gao, B.; Fan, J.; Lu, W.; Hu, Y.; Jie, Q.; et al. Suppressing UPR-dependent overactivation of FGFR3 signaling ameliorates SLC26A2-deficient chondrodysplasias. EBioMedicine 2019, 40, 695–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, A.; Sankilampi, U.; Hannila, M.-L.; Kiviniemi, V.; Kesseli, K.; Dunkel, L. New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann. Med. 2010, 43, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kausar, M.; Mäkitie, R.E.; Toiviainen-Salo, S.; Ignatius, J.; Anees, M.; Mäkitie, O. Recessive multiple epiphyseal dysplasia—Clinical characteristics caused by rare compound heterozygous SLC26A2 genotypes. Eur. J. Med. Genet. 2019, 62, 103573. [Google Scholar] [CrossRef] [Green Version]
- Syvänen, J.; Helenius, I.; Hero, M.; Mäkitie, O.; Ignatius, J. Recessive MED with auricular swelling due to compound heterozygosity Arg279Tpr/Thr512Lys in the SLC26A2 gene. Am. J. Med. Genet. Part A 2013, 161, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- García, M.M.; Velez, C.; Fenollar-Cortés, M.; Bustamante, A.; Lorda-Sánchez, I.; Soriano-Guillén, L.; Trujillo-Tiebas, M.-J. Paternal isodisomy of chromosome 5 in a patient with recessive multiple epiphyseal dysplasia. Am. J. Med. Genet. Part A 2014, 164, 1075–1078. [Google Scholar] [CrossRef]
- Huber, C. Sulphate transporter gene mutations in apparently isolated club foot. J. Med. Genet. 2001, 38, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Macías-Gómez, N.M.; Mégarbané, A.; Leal-Ugarte, E.; Rodríguez-Rojas, L.X.; Barros-Núñez, P. Diastrophic dysplasia and atelosteogenesis type II as expression of compound heterozygosis: First report of a Mexican patient and genotype-phenotype correlation. Am. J. Med. Genet. Part A 2004, 129A, 190–192. [Google Scholar] [CrossRef]
- Mäkitie, O.; Geiberger, S.; Horemuzova, E.; Hagenäs, L.; Moström, E.; Nordenskjöld, M.; Grigelioniene, G.; Nordgren, A. SLC26A2disease spectrum in Sweden—High frequency of recessive multiple epiphyseal dysplasia (rMED). Clin. Genet. 2014, 87, 273–278. [Google Scholar] [CrossRef]
- Dwyer, E.; Hyland, J.; Modaff, P.; Pauli, R.M. Genotype-phenotype correlation in DTDST dysplasias: Atelosteogenesis type II and diastrophic dysplasia variant in one family. Am. J. Med. Genet. Part A 2010, 152A, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Zechi-Ceide, R.M.; Moura, P.P.; Raskin, S.; Richieri-Costa, A.; Guion-Almeida, M.L. A compound heterozygote SLC26A2 mutation resulting in robin sequence, mild limbs shortness, accelerated carpal ossification, and multiple epiphysial dysplasia in two Brazilian sisters. A new intermediate phenotype between diastrophic dysplasia and recessi. Am. J. Med. Genet. Part A 2013, 161, 2088–2094. [Google Scholar] [CrossRef]
- Gatticchi, L.; Vešelényiová, D.; Miertus, J.; Maltese, P.E.; Manara, E.; Costantini, A.; Benedetti, S.; Ďurovčíková, D.; Krajcovic, J.; Bertelli, M. Recessive multiple epiphyseal dysplasia and Stargardt disease in two sisters. Mol. Genet. Genom. Med. 2021, e1630. [Google Scholar] [CrossRef]
- Hinrichs, T.; Superti-Furga, A.; Scheiderer, W.-D.; Bonafé, L.; Brenner, R.E.; Mattes, T. Recessive multiple epiphyseal dysplasia (rMED) with homozygosity for C653S mutation in the DTDST gene—Phenotype, molecular diagnosis and surgical treatment of habitual dislocation of multilayered patella: Case report. BMC Musculoskelet. Disord. 2010, 11, 110. [Google Scholar] [CrossRef]
- Czarny-Ratajczak, M.; Bieganski, T.; Rogala, P.; Glowacki, M.; Trzeciak, T.; Kozlowski, K. New intermediate phenotype between MED and DD caused by compound heterozygous mutations in the DTDST gene. Am. J. Med. Genet. Part A 2010, 152A, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, Y.; Zhou, H.; Liao, Z.; Gao, B.; Su, D.; Zheng, S.; Xu, C.; Su, P. Dual novel mutations in SLC26A2 in two siblings with multiple epiphyseal dysplasia 4 from a Chinese family: A case report. BMC Med Genet. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, T.-J.; Kim, O.-H.; Lee, H.-R.; Shin, S.J.; Yoo, W.J.; Park, W.Y.; Park, S.S.; Cho, S.I.; Choi, I.H. Autosomal Recessive Multiple Epiphyseal Dysplasia in a Korean Girl Caused by Novel Compound Heterozygous Mutations in the DTDST (SLC26A2) Gene. J. Korean Med. Sci. 2010, 25, 1105–1108. [Google Scholar] [CrossRef]
- Barreda-Bonis, A.C.; Barraza-García, J.; Parron, M.; Pastor, I.; Heath, K.E.; González-Casado, I. Multiple SLC26A2 mutations occurring in a three-generational family. Eur. J. Med. Genet. 2018, 61, 24–28. [Google Scholar] [CrossRef]
- Miyake, A.; Nishimura, G.; Futami, T.; Ohashi, H.; Chiba, K.; Toyama, Y.; Furuichi, T.; Ikegawa, S. A compound heterozygote of novel and recurrent DTDST mutations results in a novel intermediate phenotype of Desbuquois dysplasia, diastrophic dysplasia, and recessive form of multiple epiphyseal dysplasia. J. Hum. Genet. 2008, 53, 764–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonafe, L.; Hastbacka, J.; De La Chapelle, A.; Campos-Xavier, A.B.; Chiesa, C.; Forlino, A.; Superti-Furga, A.; Rossi, A. A novel mutation in the sulfate transporter gene SLC26A2 (DTDST) specific to the Finnish population causes de la Chapelle dysplasia. J. Med. Genet. 2008, 45, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Gualeni, B.; Facchini, M.; De Leonardis, F.; Tenni, R.; Cetta, G.; Viola, M.; Passi, A.; Superti-Furga, A.; Forlino, A.; Rossi, A. Defective proteoglycan sulfation of the growth plate zones causes reduced chondrocyte proliferation via an altered Indian hedgehog signalling. Matrix Biol. 2010, 29, 453–460. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, F.; Monti, L.; Gualeni, B.; Tenni, R.; Forlino, A.; Rossi, A. Altered Signaling in the G1 Phase Deregulates Chondrocyte Growth in a Mouse Model With Proteoglycan Undersulfation. J. Cell. Biochem. 2014, 115, 1779–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlstedt, E.; Kaitila, I.; Pirinen, S. Phenotypic features of dentition in diastrophic dysplasia. J. Craniof. Genet. Dev. Boil. 1996, 16, 164–173. [Google Scholar]
- Karlstedt, E.; Kaitila, I.; Pirinen, S. Craniofacial structure in diastrophic dysplasia—A cephalometric study. Am. J. Med. Genet. 1997, 72, 266–274. [Google Scholar] [CrossRef]
- Rintala, A.; Marttinen, E.; Rantala, S.-L.; Kaitila, I. Cleft Palate in Diastrophic Dysplasia. Scand. J. Plast. Reconstr. Surg. 1986, 20, 45–49. [Google Scholar] [CrossRef]
- Poussa, M.; Merikanto, J.; Ryöppy, S.; Marttinen, E.; Kaitila, I. The Spine in Diastrophic Dysplasia. Spine 1991, 16, 881–887. [Google Scholar] [CrossRef]
- Remes, V.; Marttinen, E.; Poussa, M.; Kaitila, I.; Peltonen, J. Cervical Kyphosis in Diastrophic Dysplasia. Spine 1999, 24, 1990. [Google Scholar] [CrossRef]
- Remes, V.M.; Marttinen, E.J.; Poussa, M.S.; Helenius, I.J.; Peltonen, J.I. Cervical spine in patients with diastrophic dysplasia—Radiographic findings in 122 patients. Pediatr. Radiol. 2002, 32, 621–628. [Google Scholar] [CrossRef]
- Potaczek, T.; Jasiewicz, B.; Duda, S.; Tesiorowski, M. Cervical spine surgery in patients with diastrophic dysplasia: Case report with long-term follow-up. J. Craniovert. Junction Spine 2015, 6, 216–218. [Google Scholar] [CrossRef]
- Remes, V.; Poussa, M.; Peltonen, J. Scoliosis in Patients with Diastrophic Dysplasia. Spine 2001, 26, 1689–1697. [Google Scholar] [CrossRef]
- Remes, V.; Tervahartiala, P.; Poussa, M.; Peltonen, J. Thoracic and Lumbar Spine in Diastrophic Dysplasia. Spine 2001, 26, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Remes, V.M.; Hästbacka, J.R.; Poussa, M.S.; Peltonen, J.I. Does genotype predict development of the spinal deformity in patients with diastrophic dysplasia? Eur. Spine J. 2002, 11, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.A.; Scott, C.I.; Hall, J.G.; Murdoch, J.L.; Mckusick, V.A. Diastrophic dwarfism. Medicine 1972, 51, 41–59. [Google Scholar] [CrossRef]
- Peltonen, J.; Remes, V.; Tervahartiala, P. Early Degeneration of the Knee in Diastrophic Dysplasia. J. Pediatr. Orthop. 2003, 23, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, J.; Vaara, P.; Marttinen, E.; Ryöppy, S.; Poussa, M. The knee joint in diastrophic dysplasia. JBJS 1999, 81, 625–631. [Google Scholar] [CrossRef]
- Bayhan, I.A.; Er, M.S.; Nishnianidze, T.; Ditro, C.; Rogers, K.J.; Miller, F.; Mackenzie, W.G. Gait Pattern and Lower Extremity Alignment in Children With Diastrophic Dysplasia. J. Pediatr. Orthop. 2016, 36, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Karniski, L.P. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: Correlation between sulfate transport activity and chondrodysplasia phenotype. Hum. Mol. Genet. 2001, 10, 1485–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient | SLC26A2 Variant 1 | SLC26A2 Variant 2 | Clinical Phenotype | Length at Birth (SDS) | Length at Age 1 (SDS) | Height at Age 5 (SDS) |
|---|---|---|---|---|---|---|
| 1 | N/A | N/A | DTD | −4.2 | N/A | N/A |
| 2 | c.-26+2T>C | c.-26+2T>C | DTD | −4.0 | −6.9 | −5.6 |
| 3 | c.-26+2T>C | c.-26+2T>C | DTD | −5.9 | −7.4 | −7.2 |
| 4 | c.-26+2T>C | c.-26+2T>C | DTD | −5.3 | −7.6 | −5.4 |
| 5 | c.-26+2T>C | c.-26+2T>C | DTD | −2.9 | −6.5 | −5.1 |
| 6 | c.-26+2T>C | c.-26+2T>C | DTD | −3.5 | −5.4 | −4.7 |
| 7 | c.-26+2T>C | c.-26+2T>C | DTD | −3.7 | −4.5 | N/A |
| 8 * | c.-26+2T>C | c.-26+2T>C | DTD | N/A | N/A | N/A |
| 9 | c.-26+2T>C | c.-26+2T>C | DTD | −3.5 | −5.3 | −2.9 |
| 10 * | c.-26+2T>C | c.-26+2T>C | DTD | −6.3 | −9.2 | −7.9 |
| 11 | N/A | N/A | DTD | −4.4 | −6.2 | N/A |
| 12 | c.-26+2T>C | N/A | DTD | N/A | N/A | −4.0 |
| 13 | Arg279Trp | Thr512Lys | rMED | −2.2 | −2.6 | −2.9 |
| 14 | Arg279Trp | Thr512Lys | rMED | −1.1 | −0.3 | N/A |
| Boys | Girls | |
|---|---|---|
| DTD | n = 4 | n = 6 |
| Length (cm) | 42.5 (39–44.5) | 43 (39.5–45) |
| Weight (g) | 3310 (2615–3570) | 3390 (2525–3900) |
| Head circumference (cm) | 36.5 (35–37) | 35.5 (32–38) |
| rMED | n = 2 | n = 0 |
| Length (cm) | 48 (47–49) | |
| Weight (g) | 3680 (3230–4120) | |
| Head circumference (cm) | 36 (35–37) |
| Clinical Features | All (n = 14) | DTD (n = 12) | rMED (n = 2) |
|---|---|---|---|
| Hand abnormalities | 100% | 100% | 100% |
| Cleft palate | 64% | 67% | 50% |
| Naevus flammeus | 21% | 25% | 0% |
| Small chin | 71% | 75% | 50% |
| Auricular abnormality | 36% | 33% | 50% |
| Club foot | 57% | 58% | 50% |
| Other foot deformity | 21% | 17% | 50% |
| ACL absence/laxity | 71% | 75% | 50% |
| Lateral position of patella | 79% | 83% | 50% |
| Patellar luxation | 57% | 58% | 50% |
| Valgus deformity | 86% | 83% | 100% |
| Cervical kyphosis | 79% | 83% | 50% |
| Scoliosis | 36% | 33% | 50% |
| Lumbar lordosis | 57% | 58% | 50% |
| SLC26A2 Variant 1 | SLC26A2 Variant 2 | Phenotype | n | Brachy-dactyly | Other Hand Deformities | Cleft Palate | Auricular Abnormality | Club Foot | Other Foot Deformity | Valgus Deformity | Patellar Luxation | Cervical Kyphosis | Scoliosis | Lumbar Lordosis | Reported in |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg279Trp | Arg279Trp | rMED | 27 | 15% | 30% | 7% | 4% | 41% | 7% | 4% | 4% | 7% | [13,17,32,33] | ||
| Arg279Trp | Arg178Ter | DTD | 8 | 88% | 100% | 25% | 100% | 88% | 13% | 88% | 63% | 38% | [13,34] | ||
| c.-26+2T>C | Arg279Trp | rMED | 4 | 25% | 25% | 25% | 0% | 25% | 25% | 50% | 75% | [35] | |||
| c.-26+2T>C | Arg279Trp | Intermediate | 4 | 75% | 75% | 50% | 50% | 50% | 25% | 50% | 25% | 25% | [36,37] | ||
| Cys653Ser | Cys653Ser | rMED | 6 | 83% | 17% | 17% | 50% | 50% | [18,38,39] | ||||||
| Cys653Ser | Ala715Val | Intermediate | 3 | 100% | 100% | 0% | 0% | 67% | 33% | 33% | 67% | 67% | [40] | ||
| Arg279Trp | c.727-1G>C | Intermediate | 2 | 100% | 100% | 0% | 100% | 100% | 100% | 50% | 50% | [13] | |||
| Leu275Pro | Leu400Phe | rMED | 2 | 50% | 0% | 50% | 50% | [41] | |||||||
| Val162fs | Asp385Asn | rMED | 1 | 100% | 0% | 0% | 100% | 100% | 0% | 0% | 0% | [42] | |||
| c.-26+2T>C | c.-26+2T>C | DTD | 1 | 100% | 100% | 100% | 100% | [35] | |||||||
| Arg279Trp | Asn425Asp | DTD | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | [13] | |||
| Arg279Trp | Ser522Phe | rMED | 1 | 100% | 0% | 100% | 100% | [43] | |||||||
| Thr266Ile | Val340del | Intermediate | 1 | 100% | 100% | 100% | [44] | ||||||||
| Arg279Trp | Thr512Lys | rMED | 1 | 0% | 100% | 100% | 0% | 0% | 0% | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Härkönen, H.; Loid, P.; Mäkitie, O. SLC26A2-Associated Diastrophic Dysplasia and rMED—Clinical Features in Affected Finnish Children and Review of the Literature. Genes 2021, 12, 714. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12050714
Härkönen H, Loid P, Mäkitie O. SLC26A2-Associated Diastrophic Dysplasia and rMED—Clinical Features in Affected Finnish Children and Review of the Literature. Genes. 2021; 12(5):714. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12050714
Chicago/Turabian StyleHärkönen, Helmi, Petra Loid, and Outi Mäkitie. 2021. "SLC26A2-Associated Diastrophic Dysplasia and rMED—Clinical Features in Affected Finnish Children and Review of the Literature" Genes 12, no. 5: 714. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12050714






