Prediction of Eye Colour in Scandinavians Using the EyeColour 11 (EC11) SNP Set
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and DNA Extraction
2.2. Quantitative Eye Colour Measurements and Eye Colour Categorisation
2.3. Variant Typing (Discovery Data Set)
2.4. Selection of Variants for Eye Colour Prediction Model (Discovery Data Set)
2.5. Variant Typing (Model Data Set)
2.6. Eye Colour Prediction Modelling (Model Data Set)
3. Results
3.1. Allele Frequencies of 44 Variants in the Discovery Data Set
3.2. Selection of Variants for Eye Colour Prediction (Discovery Data Set)
3.3. Typing of Selected Variants and IrisPlex SNPs (Model Data Set)
3.4. Eye Colour Prediction Models with EC11, IrisPlex SNPs, and rs12913832
4. Discussion
4.1. The Reference Population
4.2. The SNP Set
4.3. The Reporting Strategy
4.4. DNA Phenotyping in Forensic Genetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, S.; Liu, F.; Wollstein, A.; Kovatsi, L.; Ralf, A.; Kosiniak-Kamysz, A.; Branicki, W.; Kayser, M. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Sci. Int. Genet. 2013, 7, 98–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitanya, L.; Breslin, K.; Zuñiga, S.; Wirken, L.; Pośpiech, E.; Kukla-Bartoszek, M.; Sijen, T.; de Knijff, P.; Liu, F.; Branicki, W.; et al. The HIrisPlex-S system for eye, hair and skin colour prediction from DNA: Introduction and forensic developmental validation. Forensic Sci. Int. Genet. 2018, 35, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.; Liu, F.; Ballantyne, K.N.; van Oven, M.; Lao, O.; Kayser, M. IrisPlex: A sensitive DNA tool for accurate prediction of blue and brown eye colour in the absence of ancestry information. Forensic Sci. Int. Genet. 2011, 5, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Phillips, C.; Gomez-Tato, A.; Alvarez-Dios, J.; Casares de Cal, M.; Cruz, R.; Maroñas, O.; Söchtig, J.; Fondevila, M.; Rodriguez-Cid, M.; et al. Further development of forensic eye color predictive tests. Forensic Sci. Int. Genet. 2013, 7, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, H.S.; Tvedebrink, T.; Børsting, C.; Pereira, V.; Morling, N. Ancestry prediction efficiency of the software GenoGeographer using a z-score method and the ancestry informative markers in the Precision ID Ancestry Panel. Forensic Sci. Int. Genet. 2020, 44, 102154. [Google Scholar] [CrossRef]
- Liu, F.; van Duijn, K.; Vingerling, J.R.; Hofman, A.; Uitterlinden, A.G.; Janssens, A.C.J.; Kayser, M. Eye color and the prediction of complex phenotypes from genotypes. Curr. Biol. 2009, 19, R192–R193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, R.A.; Duffy, D.L.; Zhao, Z.Z.; Leite, F.P.; Stark, M.; Hayward, N.K.; Martin, N.; Montgomery, G.W. A single SNP in an evolutionary conserved region within Intron 86 of the HERC2 gene determines human blue-brown eye color. Am. J. Hum. Genet. 2008, 82, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Eiberg, H.; Troelsen, J.T.; Nielsen, M.; Mikkelsen, A.; Mengel-From, J.; Kjaer, K.W.; Hansen, L. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum. Genet. 2008, 123, 177–187. [Google Scholar] [CrossRef]
- Visser, M.; Kayser, M.; Palstra, R.-J. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012, 22, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Pietroni, C.; Andersen, J.D.; Johansen, P.; Andersen, M.M.; Harder, S.; Paulsen, R.R.; Børsting, C.; Morling, N. The effect of gender on eye colour variation in European populations and an evaluation of the IrisPlex prediction model. Forensic Sci. Int. Genet. 2014, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.D.; Pietroni, C.; Johansen, P.; Andersen, M.M.; Pereira, V.; Børsting, C.; Morling, N. Importance of nonsynonymous OCA 2 variants in human eye color prediction. Mol. Genet. Genom. Med. 2016, 4, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Meyer, O.S.; Lunn, M.M.B.; Garcia, S.L.; Kjærbye, A.B.; Morling, N.; Børsting, C.; Andersen, J.D. Association between brown eye colour in rs12913832:GG individuals and SNPs in TYR, TYRP1, and SLC24A4. PLoS ONE 2020, 15, e0239131. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.D.; Johansen, P.; Harder, S.; Christoffersen, S.R.; Delgado, M.C.; Henriksen, S.T.; Nielsen, M.M.; Sørensen, E.; Ullum, H.; Hansen, T.; et al. Genetic analyses of the human eye colours using a novel objective method for eye colour classification. Forensic Sci. Int. Genet. 2013, 7, 508–515. [Google Scholar] [CrossRef]
- Salvo, N.M.; Janssen, K.; Kirsebom, M.K.; Meyer, O.S.; Berg, T.; Olsen, G.-H. Forensic DNA phenotyping—Predicting eye and hair colour in a Norwegian population using Verogen’s ForenSeqTM DNA Signature Prep Kit. Forensic Sci. Int. Genet. 2021. submitted. [Google Scholar]
- Meyer, O.S.; Børsting, C.; Andersen, J.D. Perception of blue and brown eye colours for forensic DNA phenotyping. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 476–477. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santosa, F.; Symes, W.W. Linear inversion of band-limited reflection seismograms. SIAM J. Sci. Stat. Comput. 1986, 7, 1307–1330. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Taylor & Francis: Abingdon, UK, 1984; ISBN 9781351460491. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 435–446. [Google Scholar]
- Walsh, S.; Wollstein, A.; Liu, F.; Chakravarthy, U.; Rahu, M.; Seland, J.H.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Vingerling, J.R.; et al. DNA-based eye colour prediction across Europe with the IrisPlex system. Forensic Sci. Int. Genet. 2012, 6, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Chaitanya, L.; Breslin, K.; Muralidharan, C.; Bronikowska, A.; Pospiech, E.; Koller, J.; Kovatsi, L.; Wollstein, A.; Branicki, W.; et al. Global skin colour prediction from DNA. Qual. Life Res. 2017, 136, 847–863. [Google Scholar] [CrossRef] [Green Version]
- Dembinski, G.M.; Picard, C.J. Evaluation of the IrisPlex DNA-based eye color prediction assay in a United States population. Forensic Sci. Int. Genet. 2014, 9, 111–117. [Google Scholar] [CrossRef]
- Salvoro, C.; Faccinetto, C.; Zucchelli, L.; Porto, M.; Marino, A.; Occhi, G.; de los Campos, G.; Vazza, G. Performance of four models for eye color prediction in an Italian population sample. Forensic Sci. Int. Genet. 2019, 40, 192–200. [Google Scholar] [CrossRef]
- Atwood, L.; Raymond, J.; Sears, A.; Bell, M.; Daniel, R. From identification to intelligence: An assessment of the suitability of forensic DNA phenotyping service providers for use in Australian law enforcement casework. Front. Genet. 2021, 11, 11. [Google Scholar] [CrossRef]
- Kayser, M. Forensic DNA phenotyping: Predicting human appearance from crime scene material for investigative purposes. Forensic Sci. Int. Genet. 2015, 18, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Mogensen, H.S.; Børsting, C.; Morling, N. Evaluation of the precision ID Ancestry Panel for crime case work: A SNP typing assay developed for typing of 165 ancestral informative markers. Forensic Sci. Int. Genet. 2017, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Tvedebrink, T.; Eriksen, P.S.; Mogensen, H.S.; Morling, N. Weight of the evidence of genetic investigations of ancestry informative markers. Theor. Popul. Biol. 2018, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]

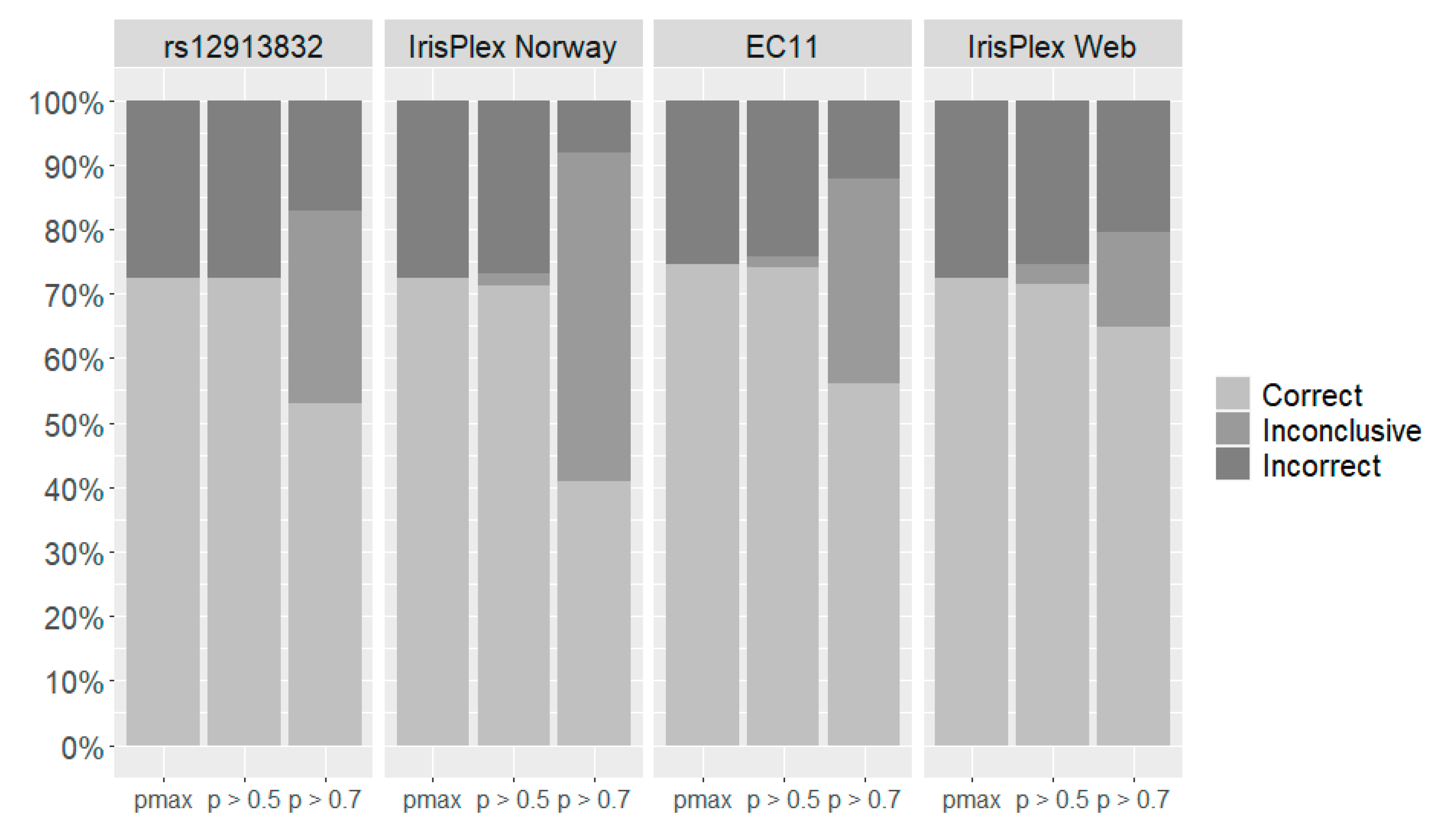
| Quantitative System 1 | Two-Category System 2 | Three-Category System 3 | ||||
|---|---|---|---|---|---|---|
| Mean PIE-Score | Blue | Brown | Blue | Intermediate | Brown | |
| Discovery data set (n = 757) | 0.24 | 447 (59%) | 310 (41%) | 368 (49%) | 148 (20%) | 241 (31%) |
| Model data set (n = 523) | 0.44 | 376 (72%) | 147 (28%) | 293 (56%) | 123 (24%) | 107 (20%) |
| Gene | Variant 1 | Reference Allele | Variant Allele | Variant Allele Frequency | |
|---|---|---|---|---|---|
| Discovery Data Set (n = 757) | Model Data Set (n = 523) | ||||
| HERC2 | rs12913832 * | A | G | 0.74 | 0.83 |
| IRF4 | rs1050976 | C | T | 0.44 | |
| IRF4 | rs10530949 | TCT | - | 0.43 | |
| IRF4 | rs12211228 | G | C | 0.14 | |
| IRF4 | rs9378807 | C | T | 0.49 | |
| TYR | rs11018509 | T | A | 0.29 | |
| TYR | rs1126809 | G | A | 0.24 | 0.24 |
| TYR | rs1393350 * | G | A | 0.23 | 0.23 |
| TYR | rs2047512 | T | C | 0.35 | |
| TYR | rs34749698 | T | C | 0.23 | |
| TYR | rs7120151 2 | A | G | 0.74 | NA |
| TYR | rs9919559 | T | C | 0.33 | |
| SLC24A4 | rs10131374 | G | A | 0.14 | 0.15 |
| TYRP1 | rs10491745 | T | C | 0.82 | |
| SLC24A4 | rs12590749 | C | A | 0.37 | |
| SLC24A4 | rs12880508 | C | T | 0.74 | |
| SLC24A4 | rs12894551 | T | C | 0.65 | |
| TYRP1 | rs1408799 | T | C | 0.68 | 0.69 |
| SLC24A4 | rs17128288 | A | G | 0.30 | |
| SLC24A4 | rs17128324 | C | T | 0.17 | |
| TYRP1 | rs201447946 | T | TA | 0.06 | |
| SLC24A4 | rs34755843 | CGACTCT | - | 0.16 | |
| SLC24A4 | rs35617057 | G | T | 0.41 | |
| SLC24A4 | rs4904887 | C | G | 0.36 | |
| SLC24A4 | rs4904891 | G | C | 0.35 | |
| SLC24A4 | rs4904897 | C | T | 0.22 | |
| SLC24A4 | rs4904927 | A | G | 0.87 | 0.89 |
| SLC24A4 | rs59977926 | T | C | 0.18 | |
| TYRP1 | rs62538950 | A | T | 0.10 | |
| TYRP1 | rs62538956 | T | C | 0.11 | |
| SLC24A4 | rs7144273 | C | T | 0.49 | |
| SLC24A4 | rs7152962 | G | A | 0.23 | |
| SLC24A4 | rs7401792 | G | A | 0.62 | |
| TYRP1 | rs74606098 | C | T | 0.06 | |
| TYRP1 | rs79586719 | G | A | 0.06 | |
| OCA2 | rs1800407 * | C | T | 0.07 | 0.03 |
| IRF4 | rs12203592 * | C | T | 0.08 | 0.09 |
| SLC24A4 | rs12896399 * | G | T | 0.49 | 0.52 |
| SLC45A2 | rs16891982 * | C | G | 0.93 | 0.95 |
| OCA2 | rs1800401 | G | A | 0.04 | 0.05 |
| OCA2 | rs1800414 | T | C | <0.01 | |
| OCA2 | rs62008729 | C | T | 0.09 | |
| OCA2 | rs121918166 2 | C | T | <0.01 | 0.01 |
| OCA2 | rs74653330 2 | C | T | <0.01 | 0.02 |
| Rank | Gene | Variant | Mean Variable Importance |
|---|---|---|---|
| 1 | HERC2 | rs12913832 * | 74.63% |
| 2 | OCA2 | rs121918166 + rs74653330 | 8.54% |
| 3 | SLC45A2 | rs16891982 * | 6.23% |
| 4 | OCA2 | rs1800407 * | 5.26% |
| 5 | TYRP1 | rs1408799 | 1.54% |
| 6 | SLC24A4 | rs4904927 | 1.19% |
| 7 | SLC24A4 | rs12896399 * | 0.68% |
| 8 | TYR | rs1126809 | 0.47% |
| 9 | TYR | rs7120151 1 | 0.46% |
| 10 | SLC24A4 | rs10131374 | 0.32% |
| 11 | OCA2 | rs1800401 | 0.32% |
| Eye Colour Prediction Model | Quantitative System 1 | Two-Category System 2 | Three-Category System 3 |
|---|---|---|---|
| EC11 | 5.07 | 0.26 | 0.59 |
| IrisPlex Norway | 5.90 | 0.30 | 0.66 |
| rs12913832 | 6.96 | 0.32 | 0.69 |
| IrisPlex Web * | NA | NA | 0.80 |
| Two-Category System | Sensitivity 1 | Specificity 1 |
|---|---|---|
| rs12913832 | 0.92 | 0.84 |
| IrisPlex Norway | 0.92 | 0.84 |
| EC11 | 0.96 | 0.82 |
| Three-Category System | Sensitivity 1 | Specificity 1 | |
|---|---|---|---|
| rs12913832 | Blue | 0.95 | 0.61 |
| Intermediate | 0.00 | 1.00 | |
| Brown | 0.95 | 0.87 | |
| IrisPlex Norway | Blue | 0.94 | 0.61 |
| Intermediate | 0.10 | 0.97 | |
| Brown | 0.86 | 0.90 | |
| EC11 | Blue | 0.95 | 0.59 |
| Intermediate | 0.15 | 0.95 | |
| Brown | 0.88 | 0.96 | |
| IrisPlex Web | Blue | 0.95 | 0.60 |
| Intermediate | 0.00 | 1.00 | |
| Brown | 0.95 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, O.S.; Salvo, N.M.; Kjærbye, A.; Kjersem, M.; Andersen, M.M.; Sørensen, E.; Ullum, H.; Janssen, K.; Morling, N.; Børsting, C.; et al. Prediction of Eye Colour in Scandinavians Using the EyeColour 11 (EC11) SNP Set. Genes 2021, 12, 821. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060821
Meyer OS, Salvo NM, Kjærbye A, Kjersem M, Andersen MM, Sørensen E, Ullum H, Janssen K, Morling N, Børsting C, et al. Prediction of Eye Colour in Scandinavians Using the EyeColour 11 (EC11) SNP Set. Genes. 2021; 12(6):821. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060821
Chicago/Turabian StyleMeyer, Olivia Strunge, Nina Mjølsnes Salvo, Anne Kjærbye, Marianne Kjersem, Mikkel Meyer Andersen, Erik Sørensen, Henrik Ullum, Kirstin Janssen, Niels Morling, Claus Børsting, and et al. 2021. "Prediction of Eye Colour in Scandinavians Using the EyeColour 11 (EC11) SNP Set" Genes 12, no. 6: 821. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12060821






