The Roles of NDR Protein Kinases in Hippo Signalling
Abstract
:1. Introduction
2. Review
2.1. The Regulation of Mammalian NDR1/2 Kinases
2.2. Biological Functions of Mammalian NDR1/2 Kinases
2.2.1. Roles of NDR1/2 in Cell Cycle Progression and Cell Cycle Associated Processes
2.2.2. Roles of NDR1/2 in Apoptosis, Stress Signalling and Autophagy
2.2.3. Roles of NDR1/2 in DNA Damage Signalling
2.2.4. Roles of NDR1/2 in Immunology
2.2.5. Roles of NDR1/2 in Neurobiology
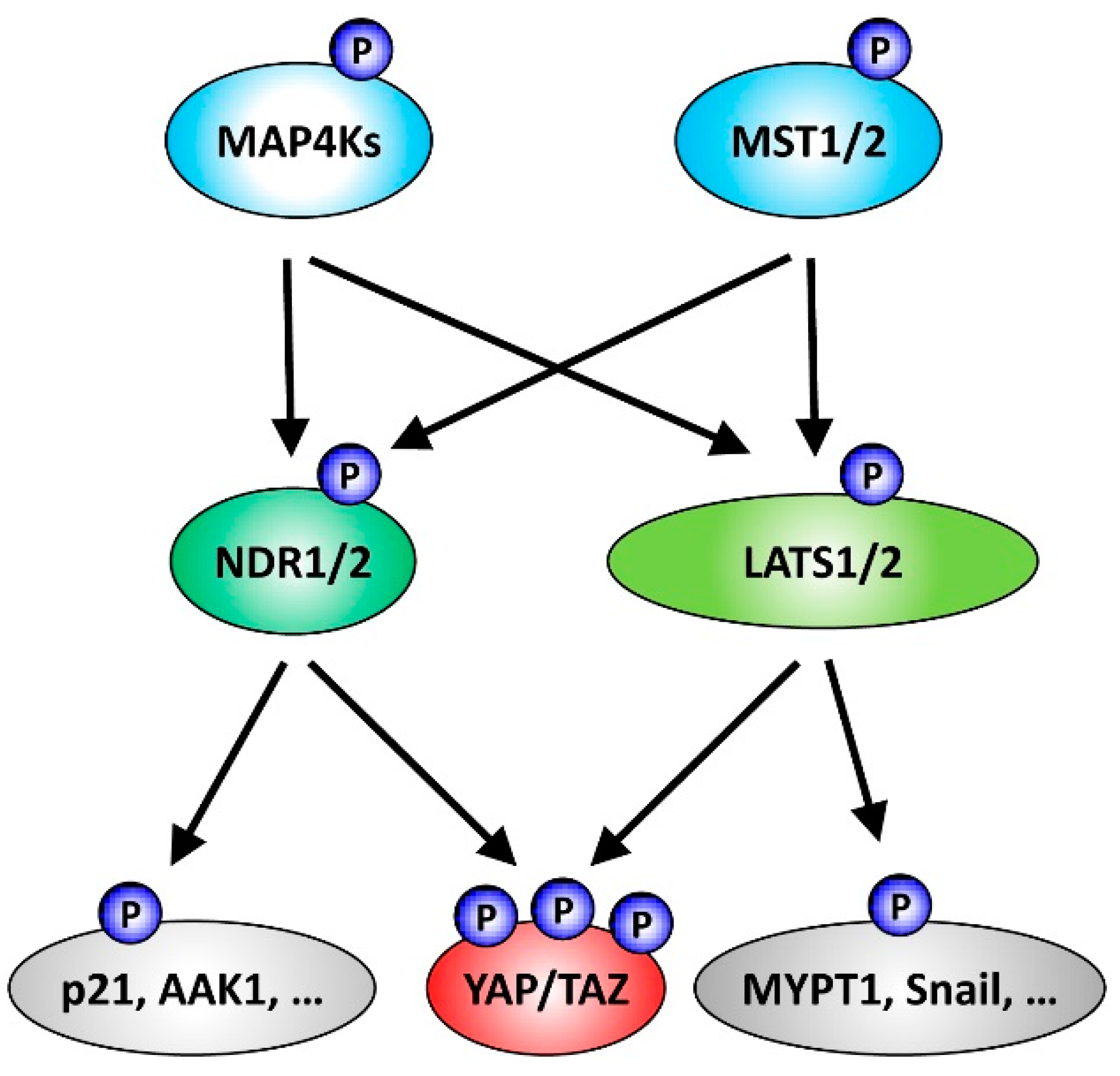
2.3. The Connections between NDR Kinases and Hippo Core Signalling
2.3.1. NDR Kinases as YAP Regulators
2.3.2. NDR Kinases Functioning Downstream of Hippo and Hippo-Like Kinases
2.3.3. NDR Kinases Regulated by the MOB1 Scaffold
2.3.4. Comparison of NDR1/2 and LATS1/2 Regulatory Mechanisms in Hippo Signalling
3. Conclusions and Future Outlook
Abbreviations
| AGC | protein kinase A (PKA)/PKG/PKC-like |
| Cip1 | cyclin-dependent kinase interacting protein 1 |
| HP1 | Heterochromatin protein 1 |
| HSP90 | Heat-shock protein 90 |
| LATS | large tumour suppressor protein kinase |
| MAP4K | Mitogen-activated protein kinase kinase kinase kinase |
| MICAL-1 | molecule interacting with CasL-1 |
| MOB1 | Mps one binder 1 |
| MST | Mammalian serine/threonine sterile 20 (Ste20)-like kinase |
| NDR | nuclear Dbf2-related protein kinase |
| Par3 | Partitioning defective 3 |
| PLK1 | Polo-like kinase 1 |
| PP2A | protein phosphatase 2A |
| RASSF | RAS association domain family |
| STK | serine/threonine protein kinase |
| TAZ | transcriptional coactivator with PDZ binding motif |
| TGF | transforming growth factor |
| Trc | Tricornered |
| ULK1 | Unc-51 like protein kinase 1 |
| YAP | Yes-associated protein |
| Yki | Yorkie |
Acknowledgments
Conflicts of Interest
References
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Stegert, M.R.; Schmitz, D.; Hemmings, B.A. NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 2006, 7, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guan, K.L. The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 2012, 23, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Mottier-Pavie, V.; Plouffe, S.W.; Hansen, C.G.; Hong, A.W.; Park, H.W.; Mo, J.S.; Lu, W.; Lu, S.; et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.; Liu, B.; Deng, H.; Uster, E.; Pan, D. Identification of happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev. Cell 2015, 34, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, S.; Mana-Capelli, S.; Roth Flach, R.J.; Danai, L.V.; Amcheslavsky, A.; Nie, Y.; Kaneko, S.; Yao, X.; Chen, X.; et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev. Cell 2014, 31, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, F.; Terracciano, L.; Hynx, D.; Kohler, R.; Bichet, S.; Hess, D.; Cron, P.; Hemmings, B.A.; Hergovich, A.; et al. NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr. Biol. 2015, 25, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.H.; Eberly, S.L.; Chapman, J.W.; Araki, H.; Sugino, A. The product of the saccharomyces cerevisiae cell cycle gene DBF2 has homology with protein kinases and is periodically expressed in the cell cycle. Mol. Cell Biol. 1990, 10, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Yarden, O.; Plamann, M.; Ebbole, D.J.; Yanofsky, C. Cot-1, a gene required for hyphal elongation in neurospora crassa, encodes a protein kinase. Embo. J. 1992, 11, 2159–2166. [Google Scholar] [PubMed]
- Millward, T.; Cron, P.; Hemmings, B.A. Molecular cloning and characterization of a conserved nuclear serine(threonine) protein kinase. Proc. Natl. Acad. Sci. USA 1995, 92, 5022–5026. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; He, B.; Wang, M.; Adler, P.N. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics 2000, 156, 1817–1828. [Google Scholar] [PubMed]
- He, Y.; Fang, X.; Emoto, K.; Jan, Y.N.; Adler, P.N. The tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with furry during Drosophila wing hair development. Mol. Biol. Cell 2005, 16, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Benz, C.; Grimaldi, R.; Stockdale, C.; Wyatt, P.; Frearson, J.; Hammarton, T.C. Nuclear DBF-2-related kinases are essential regulators of cytokinesis in bloodstream stage Trypanosoma brucei. J. Biol. Chem. 2010, 285, 15356–15368. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Rohmer, D.; Probst, S.; Yang, Z.Z.; Laurent, F.; Stadler, M.B.; Zuniga, A.; Zeller, R.; Hynx, D.; Hemmings, B.A.; Hergovich, A. NDR kinases are essential for somitogenesis and cardiac looping during mouse embryonic development. PLoS ONE 2015, 10, e0136566. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zheng, Y.; Hara, M.; Pan, D.; Luo, X. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev. 2015, 29, 1416–1431. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.; Kulaberoglu, Y.; Gundogdu, R.; Cook, D.; Mavis, M.; Gomez, M.; Gomez, V.; Hergovich, A. The characterisation of LATS2 kinase regulation in Hippo-YAP signalling. Cell Signal. 2016, 28, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A. Regulation and functions of mammalian LATS/NDR kinases: Looking beyond canonical Hippo signalling. Cell Biosci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A. Mob control: Reviewing a conserved family of kinase regulators. Cell Signal. 2011, 23, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Millward, T.A.; Hess, D.; Hemmings, B.A. NDR protein kinase is regulated by phosphorylation on two conserved sequence motifs. J. Biol. Chem. 1999, 274, 33847–33850. [Google Scholar] [CrossRef] [PubMed]
- Bichsel, S.J.; Tamaskovic, R.; Stegert, M.R.; Hemmings, B.A. Mechanism of activation of NDR (nuclear DBF2-related) protein kinase by the hMOB1 protein. J. Biol. Chem. 2004, 279, 35228–35235. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Bichsel, S.J.; Hemmings, B.A. Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol. Cell Biol. 2005, 25, 8259–8272. [Google Scholar] [CrossRef] [PubMed]
- Stegert, M.R.; Tamaskovic, R.; Bichsel, S.J.; Hergovich, A.; Hemmings, B.A. Regulation of NDR2 protein kinase by multi-site phosphorylation and the s100b calcium-binding protein. J. Biol. Chem. 2004, 279, 23806–23812. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Hoa, L.Y.; Gomez, V.; Gomez, M.; Hergovich, A. Constitutively active NDR1-PIF kinase functions independent of MST1 and hMOB1 signalling. Cell Signal. 2014, 26, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Inoue, S.; Yokosawa, H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem. Biophys. Res. Commun. 2006, 348, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Cornils, H.; Kohler, R.S.; Hergovich, A.; Hemmings, B.A. Downstream of human NDR kinases: Impacting on c-myc and p21 protein stability to control cell cycle progression. Cell Cycle 2011, 10, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Cornils, H.; Kohler, R.S.; Hergovich, A.; Hemmings, B.A. Human NDR kinases control G(1)/S cell cycle transition by directly regulating p21 stability. Mol. Cell. Biol. 2011, 31, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Bisikirska, B.C.; Adam, S.J.; Alvarez, M.J.; Rajbhandari, P.; Cox, R.; Lefebvre, C.; Wang, K.; Rieckhof, G.E.; Felsher, D.W.; Califano, A. STK38 is a critical upstream regulator of myc’s oncogenic activity in human B-cell lymphoma. Oncogene 2013, 32, 5283–5291. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Saito, M.; Bisikirska, B.C.; Alvarez, M.J.; Lim, W.K.; Rajbhandari, P.; Shen, Q.; Nemenman, I.; Basso, K.; Margolin, A.A.; et al. Genome-wide identification of post-translational modulators of transcription factor activity in human B cells. Nat. Biotechnol. 2009, 27, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Tong, X.; Ye, X. Cyclin D1 promotes cell cycle progression through enhancing NDR1/2 kinase activity independent of cyclin-dependent kinase 4. J. Biol. Chem. 2013, 288, 26678–26687. [Google Scholar] [CrossRef] [PubMed]
- Pot, I.; Patel, S.; Deng, L.; Chandhoke, A.S.; Zhang, C.; Bonni, A.; Bonni, S. Identification of a novel link between the protein kinase NDR1 and TGFβ signaling in epithelial cells. PLoS ONE 2013, 8, e67178. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Kim, M.J.; Song, S.J.; Kim, T.; Lee, D.; Kwon, S.H.; Choi, E.J.; Lim, D.S. MST1 limits the kinase activity of aurora b to promote stable kinetochore-microtubule attachment. Curr. Biol. 2010, 20, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Ikeda, M.; Katsunuma, K.; Ohashi, K.; Mizuno, K. MST2- and furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr. Biol. 2009, 19, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Prasanth, K.V.; Prasanth, S.G. Dynamic phosphorylation of HP1α regulates mitotic progression in human cells. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chu, L.; Qin, B.; Wang, Z.; Liu, X.; Jin, C.; Zhang, G.; Gomez, M.; Hergovich, A.; Chen, Z.; et al. Regulation of NDR1 activity by PLK1 ensures proper spindle orientation in mitosis. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Lamla, S.; Nigg, E.A.; Hemmings, B.A. Centrosome-associated NDR kinase regulates centrosome duplication. Mol. Cell 2007, 25, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Kohler, R.S.; Schmitz, D.; Vichalkovski, A.; Cornils, H.; Hemmings, B.A. The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr. Biol. 2009, 19, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Amagai, Y.; Homma, Y.; Fukuda, M.; Mizuno, K. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. Embo. J. 2013, 32, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, O.; Kukekova, A.V.; Aguirre, G.D.; Acland, G.M. Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 2010, 96, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Berta, A.I.; Boesze-Battaglia, K.; Genini, S.; Goldstein, O.; O’Brien, P.J.; Szel, A.; Acland, G.M.; Beltran, W.A.; Aguirre, G.D. Photoreceptor cell death, proliferation and formation of hybrid rod/S-cone photoreceptors in the degenerating STK38L mutant retina. PLoS ONE 2011, 6, e24074. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, K.I.; Johnson, C.T.; Jackson, P.K. The primary cilium as a cellular receiver: Organizing ciliary GPCR signaling. Curr. Opin. Cell Biol. 2016, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Rohatgi, R. G-protein-coupled receptors, hedgehog signaling and primary cilia. Semin. Cell Dev. Biol. 2014, 33, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pampliega, O.; Cuervo, A.M. Autophagy and primary cilia: Dual interplay. Curr. Opin. Cell Biol. 2016, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dynlacht, B.D. Assembling a primary cilium. Curr. Opin. Cell Biol. 2013, 25, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Vichalkovski, A.; Gresko, E.; Cornils, H.; Hergovich, A.; Schmitz, D.; Hemmings, B.A. NDR kinase is activated by RASSF1A/MST1 in response to fas receptor stimulation and promotes apoptosis. Curr. Biol. 2008, 18, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Dupont, N.; Hoa, L.; Gomez, V.; Pardo, R.; Goncalves-Pimentel, C.; Achard, P.; Bettoun, A.; Meunier, B.; Bauvy, C.; et al. The pro-apoptotic STK38 kinase is a new beclin1 partner positively regulating autophagy. Curr. Biol. 2015, 25, 2479–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selimoglu, R.; Bettoun, A.; Joffre, C.; Meunier, B.; Parrini, M.C.; Fesquet, D.; Formstecher, E.; Cascone, I.; Hergovich, A.; Camonis, J. RalA GTPase and MAP4K4 function through NDR1 activation in stress response and apoptotic signaling. Available online: http://www.heraldopenaccess.us/fulltext/Cell-Biology-&-Cell-Metabolism/RalA-GTPase-and-MAP4K4-Function-through-NDR1-Activation-in-Stress-Response-and-Apoptotic-Signaling.php (accessed on 18 August 2014).
- Loria, R.; Bon, G.; Perotti, V.; Gallo, E.; Bersani, I.; Baldassari, P.; Porru, M.; Leonetti, C.; Di Carlo, S.; Visca, P.; et al. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget 2015, 6, 2779–2793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Adolfs, Y.; Pijnappel, W.W.; Fuller, S.J.; Van der Schors, R.C.; Li, K.W.; Sugden, P.H.; Smit, A.B.; Hergovich, A.; Pasterkamp, R.J. MICAL-1 is a negative regulator of MST-NDR kinase signaling and apoptosis. Mol. Cell Biol. 2011, 31, 3603–3615. [Google Scholar] [CrossRef] [PubMed]
- Cornils, H.; Stegert, M.R.; Hergovich, A.; Hynx, D.; Schmitz, D.; Dirnhofer, S.; Hemmings, B.A. Ablation of the kinase NDR1 predisposes mice to the development of T cell lymphoma. Sci. Signal. 2010. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.J.; Pikkarainen, S.; Tham el, L.; Cullingford, T.E.; Molkentin, J.D.; Cornils, H.; Hergovich, A.; Hemmings, B.A.; Clerk, A.; Sugden, P.H. Nuclear Dbf2-related protein kinases (NDRs) in isolated cardiac myocytes and the myocardium: Activation by cellular stresses and by phosphoprotein serine-/threonine-phosphatase inhibitors. Cell Signal. 2008, 20, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Codogno, P.; Fanto, M.; Hergovich, A.; Camonis, J. STK38 at the crossroad between autophagy and apoptosis. Autophagy 2016, 12, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sawada, T.; Shiba, K.; Liu, S.; Kanao, T.; Takahashi, R.; Hattori, N.; Imai, Y.; Lu, B. Tricornered/NDR kinase signaling mediates pink1-directed mitochondrial quality control and tissue maintenance. Genes Dev. 2013, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kohler, R.S.; Schmitz, D.; Cornils, H.; Hemmings, B.A.; Hergovich, A. Differential NDR/LATS interactions with the human MOB family reveal a negative role for human MOB2 in the regulation of human NDR kinases. Mol. Cell Biol. 2010, 30, 4507–4520. [Google Scholar] [CrossRef] [PubMed]
- Gomez, V.; Gundogdu, R.; Gomez, M.; Hoa, L.; Panchal, N.; O’Driscoll, M.; Hergovich, A. Regulation of DNA damage responses and cell cycle progression by hMOB2. Cell Signal. 2015, 27, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Choi, J.Y.; Yi, J.M.; Chung, J.W.; Leem, S.H.; Koh, S.S.; Kang, T.H. NDR1 modulates the UV-induced DNA-damage checkpoint and nucleotide excision repair. Biochem. Biophys. Res. Commun. 2015, 461, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Fukasawa, T.; Takamatsu, N.; Ito, M.; Morita, A.; Hosoi, Y.; Miyagawa, K. The HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin modulates radiosensitivity by downregulating serine/threonine kinase 38 via Sp1 inhibition. Eur. J. Cancer. 2013, 49, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, T.; Enomoto, A.; Miyagawa, K. Serine-Threonine Kinase 38 regulates CDC25A stability and the DNA damage-induced G2/M checkpoint. Cell Signal. 2015, 27, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shirogane, T.; Xu, L.; Nalepa, G.; Qin, J.; Elledge, S.J.; Harper, J.W. SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003, 17, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Yaffe, M.B. Phospho-Ser/Thr-binding domains: Navigating the cell cycle and DNA damage response. Nat. Rev. Mol. Cell Biol. 2013, 14, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Teng, L.; Legerski, R.J. DNA damage checkpoint recovery and cancer development. Exp. Cell Res. 2015, 334, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Devroe, E.; Silver, P.A.; Engelman, A. HIV-1 incorporates and proteolytically processes human NDR1 and NDR2 serine-threonine kinases. Virology 2005, 331, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Ma, X.; Cheng, H.; Jiang, W.; Xu, X.; Zhang, Y.; Guo, Z.; Yu, Y.; Xu, H.; Qian, C.; et al. Stk38 protein kinase preferentially inhibits TLR9-activated inflammatory responses by promoting MEKK2 ubiquitination in macrophages. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Gill, J.; Ficht, X.; Barthlott, T.; Cornils, H.; Schmitz-Rohmer, D.; Hynx, D.; Zhou, D.; Zhang, L.; Xue, G.; et al. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci. Signal. 2015. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K. The growing role of the Hippo––NDR kinase signalling in neuronal development and disease. J. Biochem. 2011, 150, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K.; He, Y.; Ye, B.; Grueber, W.B.; Adler, P.N.; Jan, L.Y.; Jan, Y.N. Control of dendritic branching and tiling by the tricornered-kinase/furry signaling pathway in Drosophila sensory neurons. Cell 2004, 119, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K.; Parrish, J.Z.; Jan, L.Y.; Jan, Y.N. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 2006, 443, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Koike-Kumagai, M.; Yasunaga, K.; Morikawa, R.; Kanamori, T.; Emoto, K. The target of rapamycin complex 2 controls dendritic tiling of Drosophila sensory neurons through the tricornered kinase signalling pathway. Embo. J. 2009, 28, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Ultanir, S.K.; Hertz, N.T.; Li, G.; Ge, W.P.; Burlingame, A.L.; Pleasure, S.J.; Shokat, K.M.; Jan, L.Y.; Jan, Y.N. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in dendrite arborization and spine development. Neuron 2012, 73, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Kong, E.; Jin, J.; Hergovich, A.; Puschel, A.W. Rassf5 and NDR kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway. J. Cell Sci. 2014, 127, 3463–3476. [Google Scholar] [CrossRef] [PubMed]
- Rehberg, K.; Kliche, S.; Madencioglu, D.A.; Thiere, M.; Muller, B.; Meineke, B.M.; Freund, C.; Budinger, E.; Stork, O. The serine/threonine kinase NDR2 controls integrin trafficking and integrin-dependent neurite growth. J. Neurosci. 2014, 34, 5342–5354. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ge, M.M.; Xue, W.; Yang, Q.Q.; Wang, S.; Xu, Y.; Wang, H.L. Chronic lead exposure and mixed factors of genderxagexbrain regions interactions on dendrite growth, spine maturity and NDR kinase. PLoS ONE 2015, 10, e0138112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Mazack, V.; Sudol, M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (Yap). J. Biol. Chem. 2008, 283, 27534–27546. [Google Scholar] [CrossRef] [PubMed]
- Melixetian, M.; Klein, D.K.; Sorensen, C.S.; Helin, K. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat. Cell Biol. 2009, 11, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Adler, P.N. Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev. Biol. 2010, 341, 360–374. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Emoto, K.; Fang, X.; Ren, N.; Tian, X.; Jan, Y.N.; Adler, P.N. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol. Biol. Cell 2005, 16, 4139–4152. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008, 283, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, N.; Xie, R.; Wang, W.; Cai, J.; Choi, K.S.; David, K.K.; Huang, B.; Yabuta, N.; Nojima, H.; et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015, 29, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Meng, Z.; Kim, Y.C.; Park, H.W.; Hansen, C.G.; Kim, S.; Lim, D.S.; Guan, K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell. Biol. 2015, 17, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chang, Y.L.; Chang, Y.C.; Lin, J.C.; Chen, C.C.; Pan, S.H.; Wu, C.T.; Chen, H.Y.; Yang, S.C.; Hong, T.M.; et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.H.; Nousiainen, M.; Chalamalasetty, R.B.; Schafer, A.; Nigg, E.A.; Sillje, H.H. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005, 24, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Schmitz, D.; Hemmings, B.A. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem. Biophys. Res. Commun. 2006, 345, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, N.; Zheng, Y.; de Wilde, R.F.; Maitra, A.; Pan, D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010, 24, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 2011. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, D.; Kim, T.; Kim, T.S.; Oh, H.J.; Hwang, C.Y.; Kong, Y.Y.; Kwon, K.S.; Lim, D.S. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol. Cell. Biol. 2009, 29, 6309–6320. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Mak, K.K.; Topol, L.; Yun, K.; Hu, J.; Garrett, L.; Chen, Y.; Park, O.; Chang, J.; Simpson, R.M.; et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. USA 2010, 107, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, Y.; Kim, S.M.; Bossuyt, W.; Liu, P.; Qiu, Q.; Wang, Y.; Halder, G.; Finegold, M.J.; Lee, J.S.; et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl. Acad. Sci. USA 2010, 107, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Stegert, M.R.; Hergovich, A.; Tamaskovic, R.; Bichsel, S.J.; Hemmings, B.A. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol. Cell Biol. 2005, 25, 11019–11029. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.C.; Wei, X.; Shimizu, T.; Ramos, E.; Rohrbaugh, M.; Nikolaidis, N.; Ho, L.L.; Li, Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 2005, 120, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Devroe, E.; Erdjument-Bromage, H.; Tempst, P.; Silver, P.A. Human mob proteins regulate the NDR1 and NDR2 serine-threonine kinases. J. Biol. Chem. 2004, 279, 24444–24451. [Google Scholar] [CrossRef] [PubMed]
- Bothos, J.; Tuttle, R.L.; Ottey, M.; Luca, F.C.; Halazonetis, T.D. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005, 65, 6568–6575. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, N.; Okada, N.; Ito, A.; Hosomi, T.; Nishihara, S.; Sasayama, Y.; Fujimori, A.; Okuzaki, D.; Zhao, H.; Ikawa, M.; et al. Lats2 is an essential mitotic regulator required for the coordination of cell division. J. Biol. Chem. 2007, 282, 19259–19271. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, L.; Dumas, C.; Kajava, A.V.; Fesquet, D.; Padilla, A. NMR solution structure of Mob1, a mitotic exit network protein and its interaction with an NDR kinase peptide. J. Mol. Biol. 2004, 337, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Stavridi, E.S.; Harris, K.G.; Huyen, Y.; Bothos, J.; Verwoerd, P.M.; Stayrook, S.E.; Pavletich, N.P.; Jeffrey, P.D.; Luca, F.C. Crystal structure of a human Mob1 protein: Toward understanding Mob-regulated cell cycle pathways. Structure 2003, 11, 1163–1170. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Kuninaka, S.; Saya, H.; Lee, H.; Lim, D.S. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. Embo. J. 2013, 32, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shimizu, T.; Lai, Z.C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. Embo. J. 2007, 26, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Vrabioiu, A.M.; Struhl, G. Fat/dachsous signaling promotes Drosophila wing growth by regulating the conformational state of the NDR kinase warts. Dev. Cell 2015, 35, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Xia, F.; Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yu, J.; Zheng, Y.; Chen, Q.; Zhang, N.; Pan, D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 2013, 154, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.L.; Wei, X.; Shimizu, T.; Lai, Z.C. Mob as tumor suppressor is activated at the cell membrane to control tissue growth and organ size in Drosophila. Dev. Biol. 2010, 337, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Sugimachi, K.; Goto, H.; Wang, J.; Morikawa, T.; Miyachi, Y.; Takano, Y.; Hikasa, H.; Itoh, T.; Suzuki, S.O.; et al. Dysregulated YAP1/TAZ and TGF-β signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc. Natl. Acad. Sci. USA 2016, 113, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Hamada, K.; Kawahara, K.; Sasaki, M.; Noguchi, F.; Chiba, S.; Mizuno, K.; Suzuki, S.O.; Dong, Y.; Tokuda, M.; et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J. Clin. Investig. 2012, 122, 4505–4518. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, X.; Pfeifer, G.P. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J. Biol. Chem. 2011, 286, 6253–6261. [Google Scholar] [CrossRef] [PubMed]
- Matallanas, D.; Romano, D.; Yee, K.; Meissl, K.; Kucerova, L.; Piazzolla, D.; Baccarini, M.; Vass, J.K.; Kolch, W.; O’Neill, E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol. Cell 2007, 27, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Lee, K.K.; Song, S.J.; Jin, M.S.; Song, M.S.; Lee, J.H.; Im, C.R.; Lee, J.O.; Yonehara, S.; Lim, D.S. Role of the tumor suppressor RASSF1A in MST1-mediated apoptosis. Cancer Res. 2006, 66, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Khoklatchev, A.; Ortiz-Vega, S.; Avruch, J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 2004, 381, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kawata, A.; Nishikawa, M.; Tateishi, Y.; Yamaguchi, M.; Nakagawa, K.; Hirabayashi, S.; Bao, Y.; Hidaka, S.; Hirata, Y.; et al. Hippo pathway-dependent and -independent roles of RASSF6. Sci. Signal. 2009. [Google Scholar] [CrossRef] [PubMed]
- Huntoon, C.J.; Nye, M.D.; Geng, L.; Peterson, K.L.; Flatten, K.S.; Haluska, P.; Kaufmann, S.H.; Karnitz, L.M. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian Hippo tumor suppressor pathway. Cancer Res. 2010, 70, 8642–8650. [Google Scholar] [CrossRef] [PubMed]
- Gogl, G.; Schneider, K.D.; Yeh, B.J.; Alam, N.; Nguyen Ba, A.N.; Moses, A.M.; Hetenyi, C.; Remenyi, A.; Weiss, E.L. The structure of an NDR/LATS kinase-Mob complex reveals a novel kinase-coactivator system and substrate docking mechanism. PLoS Biol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaskovic, R.; Bichsel, S.J.; Rogniaux, H.; Stegert, M.R.; Hemmings, B.A. Mechanism of Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J. Biol. Chem. 2003, 278, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Maerz, S.; Dettmann, A.; Seiler, S. Hydrophobic motif phosphorylation coordinates activity and polar localization of the neurospora crassa nuclear Dbf2-related kinase COT1. Mol. Cell. Biol. 2012, 32, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mana-Capelli, S.; McLean, J.R.; Chen, C.T.; Ray, S.; Gould, K.L.; McCollum, D. Identification of sin pathway targets reveals mechanisms of crosstalk between NDR kinase pathways. Curr. Biol. 2013, 23, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lariviere, N.; Law, J.; Trinkle-Mulcahy, L. Dissection of a novel autocrine signaling pathway via quantitative secretome and interactome mapping. J. Proteome Res. 2014, 13, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
| Targeting motif | Target site |
|---|---|
| HVRGDpS | YAP1 (human) on Ser61 [10] |
| HSRQApS | YAP1 (human) on Ser109 [10] |
| HVRAHpS | YAP1 (human) on Ser127 [10] |
| HLRQSpS | YAP1 (human) on Ser164 [10] |
| HRRILpS | AAK1 (human) on Ser635 [72] # |
| HTRNKpS | Rabin8 (mouse) on Ser240 [72] # |
| HTRNKpS | Rabin8 (human) on Ser272 [40] |
| KRRQTpS | p21/CIP1 (human) on Ser146 [29] |
| LQRMGpS | CDC25A (human) on Ser76 [61] |
| SPGRFpS | Par3 (mouse) on Ser383 [73] |
| QSGRHpS | Par3 (human) on Ser1196 [73] |
| RKSNFpS | HP1α (human) on Ser95 [36] ## |
| HXRXXpS/T | proposed consensus motif [20] |
| Substrate | Role of phosphorylation |
|---|---|
| YAP on Ser61 | Not yet determined |
| YAP on Ser109 | Not yet determined |
| YAP on Ser127 | Facilitates cytoplasmic retention [10,76,77,78] |
| YAP on Ser164 | Not yet determined |
| AAK1 on Ser635 | Dendrite and spine development in neurons [72] |
| Rabin8 on Ser240 | Dendrite and spine development in neurons [72] |
| Rabin8 on Ser272 | Primary cilia formation [40] |
| p21/CIP1 on Ser146 | Regulates p21/CIP1 protein stability [29] |
| CDC25A on Ser76 | Regulates CDC25A protein stability [61] # |
| Par3 on Ser383 | Regulates neuronal polarity [73] |
| HP1α on Ser95 | Regulates mitotic progression [36] |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hergovich, A. The Roles of NDR Protein Kinases in Hippo Signalling. Genes 2016, 7, 21. https://0-doi-org.brum.beds.ac.uk/10.3390/genes7050021
Hergovich A. The Roles of NDR Protein Kinases in Hippo Signalling. Genes. 2016; 7(5):21. https://0-doi-org.brum.beds.ac.uk/10.3390/genes7050021
Chicago/Turabian StyleHergovich, Alexander. 2016. "The Roles of NDR Protein Kinases in Hippo Signalling" Genes 7, no. 5: 21. https://0-doi-org.brum.beds.ac.uk/10.3390/genes7050021






