Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols
Abstract
:1. Introduction
2. Current Status of Research Using DTT Assay
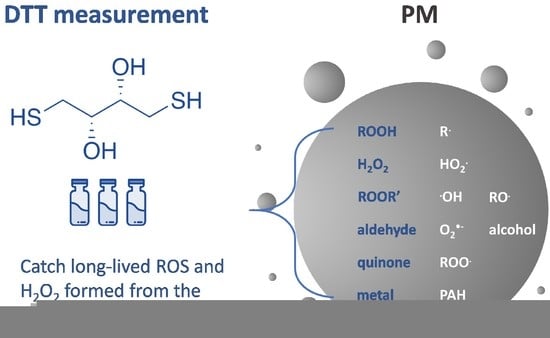
2.1. Principle of Measuring Oxidative Potential Using DTT Assay
2.2. The OPDTT of PM from Various Sources
2.3. The Oxidative Properties of Various Chemical Compositions
2.4. Recent Advancements of DTT Assay to Increase Throughput
2.5. The Correlation between Biological Responses and OPDTT
3. Additional Acellular Assays in Determination of OP
4. PM-Associated ROS: PM-Bound ROS and PM-Induced ROS
5. Challenges in Intercomparison and Interpretation of OPDTT
5.1. The Non-Standardized Protocols
5.2. The Understudied DTT Reaction Mechanisms
- (1)
- Organic hydroperoxides may interact with dissolved transition metal ions through Fenton-like reactions, leading to the formation of a variety of radical forms of reactive oxygen species including carbon and oxygen-centered organic radicals, ·OH, and O⁻₂ [72].
- (2)
- The formation of metal-organic ligand complexes may also complicate the elucidation of the DTT consumptions by PM. In the study by Yu et al., it was found that when interacting with quinones, Fe showed additive and synergistic effects in DTT consumption and ·OH, respectively, but Cu showed antagonistic effects in both measurements [54]. Meanwhile, Mn interacting with quinones showed synergistic effects in DTT consumption but antagonistic effects in ·OH generation [54]. As a comparison with the interactions with quinones, Fe, Mn, and Cu showed similar interaction pattern with HULIS, but their interactions with HULIS were weaker in DTT consumption than ·OH generation. [54]. In another study by Wei et al., Fe and Cu complex with Suwanee river fulvic acid (SRFA) showed a strong synergistic and additive effects in ROS generation, respectively [65]. DTT itself can also form specific and very stable polymeric and monomeric complexes with all of these metal ions, Zn(II), Cd(II), Pb(II), Ni(II) and Cu(I) [147].
- (3)
- Interactions among organics have been shown to affect OPDTT. For example, nitrogen-containing bases, such as pyridine, imidazole and their alkyl derivatives that are commonly found in HULIS were shown to significantly enhanced OPDTT in the presence of quinones. This observation has been attributed to the presence of unprotonated N atom in nitrogen-containing bases that can act as H-bonding acceptors to facilitate hydrogen atom transfer in the ROS generation cycle of quinones, and thus, enhance the DTT consumption [148,149].
- (4)
- High molecular weight organic compounds are commonly found in ambient PM samples. These compounds are often featured with multiple reactive functional groups within one molecule [43]. The presence of proximal reactive functional groups within high molecular weight organics could possibly influence DTT consumption, but the exact effect has not yet been fully investigated.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, C.M.S.; Jiang, H.; Chen, Y.J.; Lin, Y.-H. Traffic-related particulate matter and cardiometabolic syndrome: a review. Atmosphere 2018, 9. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef] [Green Version]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Turner Michelle, C.; Burnett Richard, T.; Jerrett, M.; Gapstur Susan, M.; Diver, W.R.; Krewski, D.; Brook Robert, D. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res. 2015, 116, 108–115. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10. [Google Scholar] [CrossRef]
- Tao, F.; Gonzalez-Flecha, B.; Kobzik, L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radical Biol. Med. 2003, 35, 327–340. [Google Scholar] [CrossRef]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. The FASEB Journal 2004, 18, 1618–1620. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef]
- Charrier, J.G.; Richards-Henderson, N.K.; Bein, K.J.; McFall, A.S.; Wexler, A.S.; Anastasio, C. Oxidant production from source-oriented particulate matter -Part 1: Oxidative potential using the dithiothreitol (DTT) assay. Atmos. Chem. Phys. 2015, 15, 2327–2340. [Google Scholar] [CrossRef]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z.D. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2014, 21, 201–210. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef] [PubMed]
- Hellack, B.; Nickel, C.; Albrecht, C.; Kuhlbusch, T.A.J.; Boland, S.; Baeza-Squiban, A.; Wohlleben, W.; Schins, R.P.F. Analytical methods to assess the oxidative potential of nanoparticles: a review. Environ. Sci. Nano 2017, 4, 1920–1934. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Guo, H.; King, L.E.; Edgerton, E.S.; Weber, R.J. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos. Meas. Tech. 2015, 8, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Cleland, W.W. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 2 - Functional targets for bioconjugation. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 127–228. [Google Scholar] [CrossRef]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Ivey, C.; Liu, C.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; et al. Source impacts on and cardiorespiratory effects of reactive oxygen species generated by water-soluble PM2.5 across the Eastern United States; Springer International Publishing: Cham, Switzerland, 2018; pp. 503–508. [Google Scholar]
- Weber, R.; Fang, T.; Verma, V. Insights on aerosol oxidative potential from measurements of particle size distributions. In Multiphase Environmental Chemistry in the Atmosphere; American Chemical Society: Washington, DC, USA, 2018; Volume 1299, pp. 417–437. [Google Scholar]
- Gao, D.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J. A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol (DTT) assay and comparisons between an urban and roadside site of water-soluble and total OP. Atmos. Meas. Tech. 2017, 10, 2821–2835. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Sioutas, C.; Weber, R.J. Oxidative properties of ambient particulate matter - an assessment of the relative contributions from various aerosol components and their emission sources. In Multiphase Environmental Chemistry in the Atmosphere; American Chemical Society: Washington, DC, USA, 2018; Volume 1299, pp. 389–416. [Google Scholar]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global perspective on the oxidative potential of airborne particulate matter: a synthesis of research findings. Environ. Sci. Technol. 2014, 48, 7576–7583. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.E.; Turner, L.R.; Benka-Coker, M.L.; Rajkumar, S.; Young, B.N.; Peel, J.L.; Clark, M.L.; Volckens, J.; Henry, C.S. Electrochemical dithiothreitol assay for large-scale particulate matter studies. Aerosol Sci. Technol. 2019, 53, 268–275. [Google Scholar] [CrossRef]
- Li, Q.F.; Wyatt, A.; Kamens, R.M. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ. 2009, 43, 1037–1042. [Google Scholar] [CrossRef]
- Rattanavaraha, W.; Rosen, E.; Zhang, H.; Li, Q.; Pantong, K.; Kamens, R.M. The reactive oxidant potential of different types of aged atmospheric particles: An outdoor chamber study. Atmos. Environ. 2011, 45, 3848–3855. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.J.; Rattanavaraha, W.; Zhang, Z.F.; Gold, A.; Surratt, J.D.; Lin, Y.H. Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol. Atmos. Environ. 2016, 130, 211–218. [Google Scholar] [CrossRef]
- Arashiro, M.; Lin, Y.-H.; Zhang, Z.; Sexton, K.G.; Gold, A.; Jaspers, I.; Fry, R.C.; Surratt, J.D. Effect of secondary organic aerosol from isoprene-derived hydroxyhydroperoxides on the expression of oxidative stress response genes in human bronchial epithelial cells. Environ. Sci. Processes Impacts 2018, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Li, X.; Wang, M.; Zhang, X.; Cao, J.; Shen, F.; Wu, Y.; Xu, S.; Fan, H.; et al. Differing toxicity of ambient particulate matter (PM) in global cities. Atmos. Environ. 2019, 212, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Gerlofs-Nijland, M.E.; Totlandsdal, A.I.; Tzamkiozis, T.; Leseman, D.L.A.C.; Samaras, Z.; Låg, M.; Schwarze, P.; Ntziachristos, L.; Cassee, F.R. Cell toxicity and oxidative potential of engine exhaust particles: impact of using particulate filter or biodiesel fuel blend. Environ. Sci. Technol. 2013, 47, 5931–5938. [Google Scholar] [CrossRef]
- Yang, J.; Roth, P.; Durbin, T.D.; Shafer, M.M.; Hemming, J.; Antkiewicz, D.S.; Asa-Awuku, A.; Karavalakis, G. Emissions from a flex fuel GDI vehicle operating on ethanol fuels show marked contrasts in chemical, physical and toxicological characteristics as a function of ethanol content. Sci. Total Environ. 2019, 683, 749–761. [Google Scholar] [CrossRef]
- McWhinney, R.D.; Zhou, S.; Abbatt, J.P.D. Naphthalene SOA: redox activity and naphthoquinone gas–particle partitioning. Atmos. Chem. Phys. 2013, 13, 9731–9744. [Google Scholar] [CrossRef]
- Jiang, H.; Jang, M.; Yu, Z. Dithiothreitol activity by particulate oxidizers of SOA produced from photooxidation of hydrocarbons under varied NOx levels. Atmos. Chem. Phys. 2017, 17, 9965–9977. [Google Scholar] [CrossRef] [Green Version]
- Riva, M.; Budisulistiorini, S.H.; Chen, Y.; Zhang, Z.; D’Ambro, E.L.; Zhang, X.; Gold, A.; Turpin, B.J.; Thornton, J.A.; Canagaratna, M.R.; et al. Chemical characterization of secondary organic aerosol from oxidation of isoprene hydroxyhydroperoxides. Environ. Sci. Technol. 2016, 50, 9889–9899. [Google Scholar] [CrossRef] [PubMed]
- Tuet, W.Y.; Chen, Y.; Xu, L.; Fok, S.; Gao, D.; Weber, R.J.; Ng, N.L. Chemical oxidative potential of secondary organic aerosol (SOA) generated from the photooxidation of biogenic and anthropogenic volatile organic compounds. Atmos. Chem. Phys. 2017, 17, 839–853. [Google Scholar] [CrossRef] [Green Version]
- Rudich, Y.; Donahue, N.M.; Mentel, T.F. Aging of organic aerosol: bridging the gap between laboratory and field studies. Annu. Rev. Phys. Chem. 2007, 58, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.L.; Canagaratna, M.R.; Jimenez, J.L.; Chhabra, P.S.; Seinfeld, J.H.; Worsnop, D.R. Changes in organic aerosol composition with aging inferred from aerosol mass spectra. Atmos. Chem. Phys. 2011, 11, 6465–6474. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Jang, M. Dynamic oxidative potential of atmospheric organic aerosol under ambient sunlight. Environ. Sci. Technol. 2018, 52, 7496–7504. [Google Scholar] [CrossRef]
- Wong, J.P.S.; Tsagkaraki, M.; Tsiodra, I.; Mihalopoulos, N.; Violaki, K.; Kanakidou, M.; Sciare, J.; Nenes, A.; Weber, R.J. Effects of atmospheric processing on the oxidative potential of biomass burning organic aerosols. Environ. Sci. Technol. 2019, 53, 6747–6756. [Google Scholar] [CrossRef]
- Wang, S.; Ye, J.; Soong, R.; Wu, B.; Yu, L.; Simpson, A.J.; Chan, A.W.H. Relationship between chemical composition and oxidative potential of secondary organic aerosol from polycyclic aromatic hydrocarbons. Atmos. Chem. Phys. 2018, 18, 3987–4003. [Google Scholar] [CrossRef] [Green Version]
- Denjean, C.; Formenti, P.; Picquet-Varrault, B.; Camredon, M.; Pangui, E.; Zapf, P.; Katrib, Y.; Giorio, C.; Tapparo, A.; Temime-Roussel, B.; et al. Aging of secondary organic aerosol generated from the ozonolysis of α-pinene: effects of ozone, light and temperature. Atmos. Chem. Phys. 2015, 15, 883–897. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Roach, P.J.; Laskin, J.; Laskin, A.; Nizkorodov, S.A. Effect of humidity on the composition of isoprene photooxidation secondary organic aerosol. Atmos. Chem. Phys. 2011, 11, 6931–6944. [Google Scholar] [CrossRef] [Green Version]
- Lambe, A.T.; Chhabra, P.S.; Onasch, T.B.; Brune, W.H.; Hunter, J.F.; Kroll, J.H.; Cummings, M.J.; Brogan, J.F.; Parmar, Y.; Worsnop, D.R.; et al. Effect of oxidant concentration, exposure time, and seed particles on secondary organic aerosol chemical composition and yield. Atmos. Chem. Phys. 2015, 15, 3063–3075. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 2010, 44, 5165–5173. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: spatiotemporal trends and source apportionment. Atmos. Chem. Phys. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Roth, P.; Ruehl, C.R.; Shafer, M.M.; Antkiewicz, D.S.; Durbin, T.D.; Cocker, D.; Asa-Awuku, A.; Karavalakis, G. Physical, chemical, and toxicological characteristics of particulate emissions from current technology gasoline direct injection vehicles. Sci. Total Environ. 2019, 650, 1182–1194. [Google Scholar] [CrossRef]
- Charrier, J.G.; McFall, A.S.; Richards-Henderson, N.K.; Anastasio, C. Hydrogen peroxide formation in a surrogate lung fluid by transition metals and quinones present in particulate matter. Environ. Sci. Technol. 2014, 48, 7010–7017. [Google Scholar] [CrossRef]
- Yu, H.; Wei, J.; Cheng, Y.; Subedi, K.; Verma, V. Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environ. Sci. Technol. 2018, 52, 2261–2270. [Google Scholar] [CrossRef]
- Shen, H.; Barakat, A.; Anastasio, C. Generation of hydrogen peroxide from San Joaquin Valley particles in a cell-free solution. Atmos. Chem. Phys. 2011, 11, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Anastasio, C. Formation of hydroxyl radical from San Joaquin Valley particles extracted in a cell-free surrogate lung fluid. Atmos. Chem. Phys. 2011, 11, 9671–9682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Jang, M.; Sabo-Attwood, T.; Robinson, S.E. Oxidative potential of secondary organic aerosols produced from photooxidation of different hydrocarbons using outdoor chamber under ambient sunlight. Atmos. Environ. 2016, 131, 382–389. [Google Scholar] [CrossRef]
- Verma, V.; Wang, Y.; El-Afifi, R.; Fang, T.; Rowland, J.; Russell, A.G.; Weber, R.J. Fractionating ambient humic-like substances (HULIS) for their reactive oxygen species activity – Assessing the importance of quinones and atmospheric aging. Atmos. Environ. 2015, 120, 351–359. [Google Scholar] [CrossRef]
- Wilson, I.; Wardman, P.; Lin, T.-S.; Sartorelli, A.C. Reactivity of thiols towards derivatives of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. Chem. Biol. Interact. 1987, 61, 229–240. [Google Scholar] [CrossRef]
- Wardman, P. Bioreductive activation of quinones: redox properties and thiol reactivity. Free Radic. Res. Commun. 1990, 8, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, F.; Wang, D.; Hasheminassab, S.; Verma, V.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Oxidative potential of on-road fine particulate matter (PM2.5) measured on major freeways of Los Angeles, CA, and a 10-year comparison with earlier roadside studies. Atmos. Environ. 2017, 148, 102–114. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; DeMinter, J.T.; Weinstein, J.P. Emissions of metals associated with motor vehicle roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Stadtman, E.R. The iron-catalyzed oxidation of dithiothreitol is a biphasic process: hydrogen peroxide is involved in the initiation of a free radical chain of reactions. Arch. Biochem. Biophys. 1996, 333, 233–242. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Wei, J.; Yu, H.; Wang, Y.; Verma, V. Complexation of iron and copper in ambient particulate matter and its effect on the oxidative potential measured in a surrogate lung fluid. Environ. Sci. Technol. 2019, 53, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.F.; Webb, P.J.; Lewis, A.C.; Reviejo, M.M. Quantifying small molecules in secondary organic aerosol formed during the photo-oxidation of toluene with hydroxyl radicals. Atmos. Environ. 2005, 39, 7263–7275. [Google Scholar] [CrossRef]
- Tuet, W.Y.; Chen, Y.; Fok, S.; Champion, J.A.; Ng, N.L. Inflammatory responses to secondary organic aerosols (SOA) generated from biogenic and anthropogenic precursors. Atmos. Chem. Phys. 2017, 2017, 11423–11440. [Google Scholar] [CrossRef]
- Chen, X.; Hopke, P.K. A chamber study of secondary organic aerosol formation by limonene ozonolysis. Indoor Air 2010, 20, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hopke, P.K. A chamber study of secondary organic aerosol formation by linalool ozonolysis. Atmos. Environ. 2009, 43, 3935–3940. [Google Scholar] [CrossRef]
- Tong, H.; Lakey, P.S.; Arangio, A.M.; Socorro, J.; Shen, F.; Lucas, K.; Brune, W.H.; Pöschl, U.; Shiraiwa, M. Reactive oxygen species formed by secondary organic aerosols in water and surrogate lung fluid. Environ. Sci. Technol. 2018, 52, 11642–11651. [Google Scholar] [CrossRef]
- Tong, H.; Lakey, P.S.J.; Arangio, A.M.; Socorro, J.; Kampf, C.J.; Berkemeier, T.; Brune, W.H.; Poschl, U.; Shiraiwa, M. Reactive oxygen species formed in aqueous mixtures of secondary organic aerosols and mineral dust influencing cloud chemistry and public health in the Anthropocene. Faraday Discuss. 2017, 200, 251–270. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kim, H.; Paulson, S.E. Hydrogen peroxide generation from α-and β-pinene and toluene secondary organic aerosols. Atmos. Environ. 2011, 45, 3149–3156. [Google Scholar] [CrossRef]
- Grek, C.L.; Zhang, J.; Manevich, Y.; Townsend, D.M.; Tew, K.D. Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 2013, 288, 26497–26504. [Google Scholar] [CrossRef]
- Chen, J.Y.; Jiang, H.; Chen, S.J.; Cullen, C.; Ahmed, C.M.S.; Lin, Y.-H. Characterization of electrophilicity and oxidative potential of atmospheric carbonyls. Environ. Sci. Processes Impacts 2019, 21, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Gant, T.W.; Ramakrishna Rao, D.N.; Mason, R.P.; Cohen, G.M. Redox cycling and sulphydryl arylation; Their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem. Biol. Interact. 1988, 65, 157–173. [Google Scholar] [CrossRef]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, J.V.; Zhang, C.; Verma, V. Development and field testing of an online instrument for measuring the real-time oxidative potential of ambient particulate matter based on dithiothreitol assay. Atmos. Meas. Tech. 2018, 11, 5767–5780. [Google Scholar] [CrossRef] [Green Version]
- Sameenoi, Y.; Koehler, K.; Shapiro, J.; Boonsong, K.; Sun, Y.; Collett, J.; Volckens, J.; Henry, C.S. Microfluidic electrochemical sensor for on-line monitoring of aerosol oxidative activity. J. Am. Chem. Soc. 2012, 134, 10562–10568. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.A.; Shapiro, J.; Sameenoi, Y.; Henry, C.; Volckens, J. Laboratory evaluation of a microfluidic electrochemical sensor for aerosol oxidative load. Aerosol Sci. Technol. 2014, 48, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Eiguren-Fernandez, A.; Kreisberg, N.; Hering, S. An online monitor of the oxidative capacity of aerosols (o-MOCA). Atmos. Meas. Tech. 2017, 10, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Abrams, J.Y.; Weber, R.J.; Klein, M.; Samat, S.E.; Chang, H.H.; Strickland, M.J.; Verma, V.; Fang, T.; Bates, J.T.; Mulholland, J.A. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ. Health Perspect. 2017, 125, 107008. [Google Scholar] [CrossRef]
- Verma, V.; Ning, Z.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 2009, 43, 6360–6368. [Google Scholar] [CrossRef]
- Karavalakis, G.; Gysel, N.; Schmitz, D.A.; Cho, A.K.; Sioutas, C.; Schauer, J.J.; Cocker, D.R.; Durbin, T.D. Impact of biodiesel on regulated and unregulated emissions, and redox and proinflammatory properties of PM emitted from heavy-duty vehicles. Sci. Total Environ. 2017, 584, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A.J.E.h.p. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Samoli, E.; Analitis, A.; Fuller, G.W.; Green, D.C.; Anderson, H.R.; Purdie, E.; Dunster, C.; Aitlhadj, L.; Kelly, F.J.; et al. Short-term associations between particle oxidative potential and daily mortality and hospital admissions in London. Int. J. Hyg. Environ. Health 2016, 219, 566–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Janssen, N.A.H.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Canepari, S.; Frasca, D.; Simonetti, G. Oxidative potential of selected PM components. In Proceedings of the 2nd International Electronic Conference on Atmospheric Sciences; MDPI: Basel, Switzerland, 2017. [Google Scholar]
- Yang, A.; Jedynska, A.; Hellack, B.; Kooter, I.; Hoek, G.; Brunekreef, B.; Kuhlbusch, T.A.J.; Cassee, F.R.; Janssen, N.A.H. Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type. Atmos. Environ. 2014, 83, 35–42. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.F.; Thomas, F.; Molton, F.; Charron, A.; Dunster, C.; Oliete, A.; et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. 2018, 18, 7863–7875. [Google Scholar] [CrossRef] [Green Version]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.-C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent advances in reactive oxygen species measurement in biological systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, D.; Wood, C.D.; Castro-Obregón, S.; Covarrubias, L. Reactive oxygen species: A radical role in development? Free Radical Biol. Med. 2010, 49, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Yu, H.; Wang, R.; Wei, J.; Verma, V. Rethinking dithiothreitol-based particulate matter oxidative potential: measuring dithiothreitol consumption versus reactive oxygen species generation. Environ. Sci. Technol. 2017, 51, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Kervinen, M.; Pätsi, J.; Finel, M.; Hassinen, I.E. Lucigenin and coelenterazine as superoxide probes in mitochondrial and bacterial membranes. Anal. Biochem. 2004, 324, 45–51. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, M.; Li, Y.; Xu, X.; Barsoum, M.; Chen, Y.; Bache, R.J. NADPH oxidase contributes to coronary endothelial dysfunction in the failing heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H840–H846. [Google Scholar] [CrossRef] [Green Version]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Datta, K.; Sinha, S.; Chattopadhyay, P. Reactive oxygen species in health and disease. Natl. Med. J. India 2000, 13, 304–310. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Finosh, G.T.; Jayabalan, M. Reactive oxygen species°™Control and management using amphiphilic biosynthetic hydrogels for cardiac applications. Adv Biosci Biotechnol. 2013, Vol.04No.12, 13. [Google Scholar] [CrossRef]
- Stephenson, G.F.; Chan, H.M.; Cherian, M.G. Copper-metallothionein from the toxic milk mutant mouse enhances lipid peroxidation initiated by an organic hydroperoxide. Toxicol. Appl. Pharmacol. 1994, 125, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Stevanovic, S.; Miljevic, B.; Bottle, S.E.; Ristovski, Z.; Kalberer, M. Quantification of particle-bound organic radicals in secondary organic aerosol. Environ. Sci. Technol. 2019, 53, 6729–6737. [Google Scholar] [CrossRef] [PubMed]
- Crilley, L.R.; Knibbs, L.D.; Miljevic, B.; Cong, X.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D.; Ayoko, G.A.; Morawska, L. Concentration and oxidative potential of on-road particle emissions and their relationship with traffic composition: Relevance to exposure assessment. Atmos. Environ. 2012, 59, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Pourkhesalian, A.M.; Stevanovic, S.; Rahman, M.M.; Faghihi, E.M.; Bottle, S.E.; Masri, A.R.; Brown, R.J.; Ristovski, Z.D. Effect of atmospheric aging on volatility and reactive oxygen species of biodiesel exhaust nano-particles. Atmos. Chem. Phys. 2015, 15, 9099–9108. [Google Scholar] [CrossRef] [Green Version]
- Stevanovic, S.; Miljevic, B.; Surawski, N.C.; Fairfull-Smith, K.E.; Bottle, S.E.; Brown, R.; Ristovski, Z.D. Influence of oxygenated organic aerosols (OOAs) on the oxidative potential of diesel and biodiesel particulate matter. Environ. Sci. Technol. 2013, 47, 7655–7662. [Google Scholar] [CrossRef] [PubMed]
- Miljevic, B.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D. The application of profluorescent nitroxides to detect reactive oxygen species derived from combustion-generated particulate matter: Cigarette smoke —A case study. Atmos. Environ. 2010, 44, 2224–2230. [Google Scholar] [CrossRef]
- Hedayat, F.; Stevanovic, S.; Milic, A.; Miljevic, B.; Nabi, M.N.; Zare, A.; Bottle, S.E.; Brown, R.J.; Ristovski, Z.D. Influence of oxygen content of the certain types of biodiesels on particulate oxidative potential. Sci. Total Environ. 2016, 545-546, 381–388. [Google Scholar] [CrossRef]
- Arangio, A.M.; Tong, H.; Socorro, J.; Pöschl, U.; Shiraiwa, M. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles. Atmos. Chem. Phys. 2016, 16, 13105–13119. [Google Scholar] [CrossRef] [Green Version]
- Gehling, W.; Dellinger, B. Environmentally persistent free radicals and their lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef]
- Epstein, S.A.; Blair, S.L.; Nizkorodov, S.A. Direct photolysis of alpha-pinene ozonolysis secondary organic aerosol: effect on particle mass and peroxide content. Environ. Sci. Technol. 2014, 48, 11251–11258. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.S.; Wu, W.; Lim, Y.B.; Ziemann, P.J. Contributions of organic peroxides to secondary aerosol formed from reactions of monoterpenes with O3. Environ. Sci. Technol. 2005, 39, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Bateman, A.P.; Bones, D.L.; Nizkorodov, S.A.; Laskin, J.; Laskin, A. High-resolution mass spectrometry analysis of secondary organic aerosol generated by ozonolysis of isoprene. Atmos. Environ. 2010, 44, 1032–1042. [Google Scholar] [CrossRef]
- Surratt, J.D.; Murphy, S.M.; Kroll, J.H.; Ng, N.L.; Hildebrandt, L.; Sorooshian, A.; Szmigielski, R.; Vermeylen, R.; Maenhaut, W.; Claeys, M.; et al. Chemical composition of secondary organic aerosol formed from the photooxidation of isoprene. J. Phys. Chem. A 2006, 110, 9665–9690. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, P.J.; Mahon, B.M.; Wragg, F.P.H.; Fuller, S.J.; Giorio, C.; Kourtchev, I.; Kalberer, M. Multiphase composition changes and reactive oxygen species formation during limonene oxidation in the new Cambridge Atmospheric Simulation Chamber (CASC). Atmos. Chem. Phys. 2017, 17, 9853–9868. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef]
- Darquenne, C. Aerosol deposition in the human lung in reduced gravity. J. Aerosol Med. Pulm. D. 2014, 27, 170–177. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819. [Google Scholar]
- Xia, T.; Kovochich, M.; Nel, A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin Occup Environ Med 2006, 5, 817–836. [Google Scholar]
- Cederbaum, A.I. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol 2003, 30, 115–120. [Google Scholar] [CrossRef]
- Kaphalia, L.; Calhoun, W.J. Alcoholic lung injury: Metabolic, biochemical and immunological aspects. Toxicol. Lett. 2013, 222, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Eeden, S.F.v. Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD. Int. J. Mol. Med. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.N.; Diaz-Sanchez, D.; Jaspers, I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J. Allergy Clin. Immunol. 2012, 129, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Roles of toll-like receptors in nitroxidative stress in mammals. Cells 2019, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Guo, Y.; Chai, J.; Shang, J.; Zhu, J.; Stevanovic, S.; Ristovski, Z. Comparison of light absorption and oxidative potential of biodiesel/diesel and chemicals/diesel blends soot particles. J. Environ. Sci. 2020, 87, 184–193. [Google Scholar] [CrossRef]
- Nishita-Hara, C.; Hirabayashi, M.; Hara, K.; Yamazaki, A.; Hayashi, M. Dithiothreitol-measured oxidative potential of size-segregated particulate matter in Fukuoka, Japan: effects of Asian dust events. GeoHealth 2019, 3, 160–173. [Google Scholar] [CrossRef]
- Lin, M.; Yu, J.Z. Dithiothreitol (DTT) concentration effect and its implications on the applicability of DTT assay to evaluate the oxidative potential of atmospheric aerosol samples. Environ. Pollut. 2019, 251, 938–944. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Y.; Qiu, X.; Cao, G.; Kuang, B.; Yu, J.Z.; Hu, D. Optical properties, source apportionment and redox activity of humic-like substance (HULIS) in airborne fine particulates in Hong Kong. Environ. Pollut. 2019, 255, 113087. [Google Scholar] [CrossRef] [PubMed]
- Eiguren-Fernandez, A.; Shinyashiki, M.; Schmitz, D.A.; DiStefano, E.; Hinds, W.; Kumagai, Y.; Cho, A.K.; Froines, J.R. Redox and electrophilic properties of vapor- and particle-phase components of ambient aerosols. Environ. Res. 2010, 110, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Verma, V.; Schauer, J.J.; Cassee, F.R.; Cho, A.K.; Sioutas, C. Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ. Sci. Tech. 2009, 43, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fne ambient aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A. Chemical composition of ambient particulate matter and redox activity. Environ. Monit. Assess. 2010, 169, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Geller, M.D.; Ntziachristos, L.; Mamakos, A.; Samaras, Z.; Schmitz, D.A.; Froines, J.R.; Sioutas, C. Physicochemical and redox characteristics of particulate matter (PM) emitted from gasoline and diesel passenger cars. Atmos. Environ. 2006, 40, 6988–7004. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, Y.; Ge, Y.; He, H.; Liu, Y. Effects of ultrasonic treatment on dithiothreitol (DTT) assay measurements for carbon materials. J. Environ. Sci. 2019, 84, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Miljevic, B.; Hedayat, F.; Stevanovic, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D. To sonicate or not to sonicate PM filters: reactive oxygen species generation upon ultrasonic iradiation. Aerosol Sci. Technol. 2014, 48, 1276–1284. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fedoseyenko, D.; Mohapatra, P.P.; Steel, P.J. Reactions of p-benzoquinone with S-nucleophiles. Synthesis 2008, 5, 777–787. [Google Scholar] [CrossRef]
- Hiemstra, H.; Wynberg, H. Addition of aromatic thiols to conjugated cycloalkenones, catalyzed by chiral. beta.-hydroxy amines. A mechanistic study of homogeneous catalytic asymmetric synthesis. J. Am. Chem. Soc. 1981, 103, 417–430. [Google Scholar] [CrossRef]
- Misra, H.P. Generation of superoxide free radical during the autoxidation of thiols. J. Biol. Chem. 1974, 249, 2151–2155. [Google Scholar] [PubMed]
- Krȩżel, A.; Leśniak, W.; Jeżowska-Bojczuk, M.; Młynarz, P.; Brasuñ, J.; Kozłowski, H.; Bal, W. Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant. J. Inorg. Biochem. 2001, 84, 77–88. [Google Scholar] [CrossRef]
- Dou, J.; Lin, P.; Kuang, B.-Y.; Yu, J.Z. Reactive oxygen species production mediated by humic-like substances in atmospheric aerosols: enhancement effects by pyridine, imidazole, and their derivatives. Environ. Sci. Technol. 2015, 49, 6457–6465. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.H.; Faraj, C.; Li, G.; Njus, D. Imidazole facilitates electron transfer from organic reductants. Bioelectrochemistry 2004, 64, 7–13. [Google Scholar] [CrossRef] [PubMed]


| Compounds | (DTT)0 a (µM) | Incubation & Shaking Method | (Sample)0 b (µM) | DTTr c (nmol/min/µg) | NIOG d | Reference |
|---|---|---|---|---|---|---|
| Formaldehyde | 20 | 37 °C, Incubator | 0.54–2.69 × 106 | 8.50 × 10−6 | 3.79 × 10−5 | Chen & Jiang et al. [75] |
| 2-Furaldehyde | 20 | 37 °C, Incubator | 1.91–9.60 × 103 | 1.05 × 10−4 | 4.69 × 10−4 | Chen & Jiang et al. [75] |
| Benzaldehyde | 20 | 37 °C, Incubator | 0.78–3.88 × 105 | 1.53 × 10−5 | 6.83 × 10−5 | Chen & Jiang et al. [75] |
| 4-Formylbenzoic acid | 20 | 37 °C, Incubator | 0.26–1.30 × 103 | 1.67 × 10−4 | 5.51 × 10−9 | Chen & Jiang et al. [75] |
| 2-Nitrobenzaldehyde | 20 | 37 °C, Incubator | 0.05–1.10 × 103 | 6.43 × 10−4 | 2.87 × 10−3 | Chen & Jiang et al. [75] |
| 3-Nitrobenzaldehyde | 20 | 37 °C, Incubator | 0.05–1.04 × 103 | 2.06 × 10−4 | 9.20 × 10−4 | Chen & Jiang et al. [75] |
| 4-Nitrobenzaldehyde | 20 | 37 °C, Incubator | 0.05–1.09 × 103 | 3.52 × 10−4 | 1.57 × 10−3 | Chen & Jiang et al. [75] |
| Mesityl oxide | 20 | 37 °C, Incubator | 0.88–4.46 × 103 | 1.02 × 10−4 | 4.55 × 10−4 | Chen & Jiang et al. [75] |
| Citral | 20 | 37 °C, Incubator | 0.30–1.51 × 103 | 1.53 × 10−4 | 6.83 × 10−4 | Chen & Jiang et al. [75] |
| trans-Cinnamaldehyde | 20 | 37 °C, Incubator | 2.82 × 104 | 1.51 × 10−3 | 6.74 × 10−3 | Chen & Jiang et al. [75] |
| 1,4-NQN | 20 | 37 °C, Incubator | 6.46 × 10−1 | 2.24 × 10−1 | 1.00 | Chen & Jiang et al. [75] |
| Isoprene epoxydiol | 20 | 37 °C, Incubator | NA | 7.00 ± 1.39 × 10−5 | 4.93 ± 0.98 × 10−5 | Kramer et al. [30] |
| 2-Methyltetrol | 20 | 37 °C, Incubator | NA | 4.44 ± 0.92 × 10−5 | 3.13 ± 0.65 × 10−5 | Kramer et al. [30] |
| Methacrylic acid epoxide | 20 | 37 °C, Incubator | NA | 9.84 ± 0.97 × 10−5 | 6.93 ± 0.68 × 10−5 | Kramer et al. [30] |
| 2-Methylglyceric acid | 20 | 37 °C, Incubator | NA | 2.51 ± 0.37 × 10−4 | 1.77 ± 0.26 × 10−4 | Kramer et al. [30] |
| ISOPOOH | 20 | 37 °C, Incubator | NA | 4.90 ± 2.20 × 10−1 | 3.45 ± 1.55 × 10−1 | Kramer et al. [30] |
| 1,4-NQN | 20 | 37 °C, Incubator | NA | 1.42 | 1.00 | Kramer et al. [30] |
| Acrolein | 100 | 37 °C, Sonicator | 5.40 × 101 | 8.60 ± 0.36 × 10−2 | 2.95 ± 0.12×10−2 | Jiang et al. [57] |
| Methacrolein | 100 | 37 °C, Sonicator | 1.77 × 102 | 3.26 ± 0.10 × 10−2 | 1.12 ± 0.03×10−2 | Jiang et al. [57] |
| 2,4-Hexadienal | 100 | 37 °C, Sonicator | 2.13 × 102 | 6.32 ± 2.39 × 10−3 | 2.16 ± 0.82×10−3 | Jiang et al. [36] |
| 9,10-PQN | 100 | 37 °C, Sonicator | 2.50 × 10−2 | 2.54 ± 0.10 × 101 | 8.72 ± 0.34 | Jiang et al. [57] |
| 1,2-NQN | 100 | 37 °C, Sonicator | 3.00 × 10−1 | 9.07 ± 0.29 | 3.11 ± 0.10 | Jiang et al. [57] |
| 1,4-NQN | 100 | 37 °C, Sonicator | 6.00 × 10−1 | 2.92 ± 0.12 | 1.00 | Jiang et al. [57] |
| tert-Butyl hydroperoxide | 100 | 37 °C, Sonicator | 280 | 1.17 ± 0.19 × 10−2 | 4.01 ± 0.65 × 10−3 | Jiang et al. [36] |
| 9,10-PQN | 100 | 37 °C, Dry bath | 0.25–2 × 10−1 | 6.77 × 101 | 2.01 × 101 | Charrier and Anastasio [49] |
| 1,2-NQN | 100 | 37 °C, Dry bath | 0.01–1 | 2.59 × 101 | 7.67 | Charrier and Anastasio [49] |
| 1,4-NQN | 100 | 37 °C, Dry bath | 0.5–1.5 | 3.37 | 1 | Charrier and Anastasio [49] |
| BQN | 100 | 37 °C, Dry bath | 1–4 | 1.17 | 0.35 | Charrier and Anastasio [49] |
| Co (II) | 100 | 37 °C, Dry bath | 1 | 4.58 | 1.36 | Charrier and Anastasio [49] |
| Ni (II) | 100 | 37 °C, Dry bath | 0.1–5 | 1.81 | 0.54 | Charrier and Anastasio [49] |
| V (V) | 100 | 37 °C, Dry bath | 1–5 | 1.98 | 0.59 | Charrier and Anastasio [49] |
| Pb (II) | 100 | 37 °C, Dry bath | 1 | 0.31 | 0.09 | Charrier and Anastasio [49] |
| Fe(II) | 100 | 37 °C, Dry bath | 0.5–5 | 0.93 | 0.28 | Charrier and Anastasio [49] |
| Fe (III) | 100 | 37 °C, Dry bath | 0.5–10 | 0.30 | 0.09 | Charrier and Anastasio [49] |
| 5-H-1,4-NQN | 100 | Room temp | NA | 7.8 | 3.7 | McWhinney et al. [35] |
| 1,2-NQN | 100 | Room temp | NA | 5.7 | 2.7 | McWhinney et al. [35] |
| 1,4-NQN | 100 | Room temp | NA | 2.1 | 1.0 | McWhinney et al. [35] |
| Aerosol System | Method | PM- BoundROS a | Sample Concentration (nmol μg−1) | Reference |
|---|---|---|---|---|
| α-Pinene SOA | BPEAnit | Radicals | 0.0200 ± 0.0050 | Campbell et al. [108] |
| Limonene SOA | BPEAnit | Radicals | 0.0059 ± 0.0010 | Campbell et al. [108] |
| β-caryophyllene | BPEAnit | Radicals | 0.0025 ± 0.00080 | Campbell et al. [108] |
| Roadside PM2.5 | BPEAnit | Radicals | 0.1−10 | Crilley et al. [109] |
| Biodiesel combustion | BPEAnit | Radicals | 0.001−1 | Pourkhesalian et al. [110] |
| Diesel combustion | BPEAnit | Radicals | 0.04 | Stevanovic et al. [111] |
| SOY biodiesel | BPEAnit | Radicals | 1.5 | Stevanovic et al. [111] |
| Side stream cigarette smoke | BPEAnit | Radicals | 0.02–0.05 | Miljevic et al. [112] |
| Biodiesel combustion | BPEAnit | Radicals | 0.05–0.4 | Hedayat et al. [113] |
| PM2.5 EPFR | EPR | Radicals | 0.2–1.0 × 10−3 | Arangio et al. [114] |
| PM2.5 water extract | EPR | Radicals | 4.0 × 10−5 | Arangio et al. [114] |
| Naphthalene SOA EPFR | EPR | Radicals | 0.02–0.05 | Tong et al. [71] |
| PM2.5 EPFR | EPR | Radicals | 0.05–0.40 | Gehling and Dellinger [115] |
| Wood smoke particles | NPBA | ROOH | 1.60–2.56 | Jiang et al. [41] |
| Gasoline LNOX SOA | NPBA | ROOH | 2.18–2.28 | Jiang et al. [41] |
| α-Pinene LNOX SOA | NPBA | ROOH | 3.81–7.34 | Jiang et al. [41] |
| Toluene LNOX SOA | NPBA | ROOH | 3.53 ± 1.90 | Jiang et al. [36] |
| Toluene HNOX SOA | NPBA | ROOH | 5.41 ± 0.73 | Jiang et al. [36] |
| Isoprene LNOX SOA | NPBA | ROOH | 2.80 ± 0.37 | Jiang et al. [36] |
| Isoprene HNOX SOA | NPBA | ROOH | 1.13 ± 0.64 | Jiang et al. [36] |
| α-Pinene + O3 SOA | KI | ROOH, ROOR | 0.79 ± 0.17 | Epstein et al. [116] |
| α-Pinene + O3 SOA | KI | ROOH, ROOR | 0.95–2.03 | Docherty et al. [117] |
| Δ-3 Carene + O3 SOA | KI | ROOH, ROOR | 0.82–1.45 | Docherty et al. [117] |
| β-Pinene + O3 SOA | KI | ROOH, ROOR | 2.42–4.00 | Docherty et al. [117] |
| Sabinene + O3 SOA | KI | ROOH, ROOR | 3.09–3.44 | Docherty et al. [117] |
| Isoprene + O3 SOA | KI | ROOH, ROOR | 1.0 ± 0.1 | Nguyen et al. [118] |
| Isoprene LNOX SOAA | KI | ROOH, ROOR | 0.80–2.06 | Surratt et al. [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Ahmed, C.M.S.; Canchola, A.; Chen, J.Y.; Lin, Y.-H. Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols. Atmosphere 2019, 10, 571. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos10100571
Jiang H, Ahmed CMS, Canchola A, Chen JY, Lin Y-H. Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols. Atmosphere. 2019; 10(10):571. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos10100571
Chicago/Turabian StyleJiang, Huanhuan, C. M. Sabbir Ahmed, Alexa Canchola, Jin Y. Chen, and Ying-Hsuan Lin. 2019. "Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols" Atmosphere 10, no. 10: 571. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos10100571