Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality
Abstract
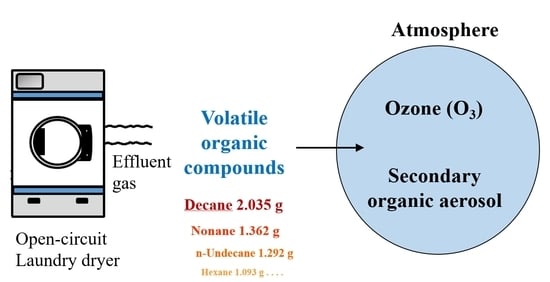
:1. Introduction
2. Materials and Methods
2.1. Dry Cleaning Process
2.2. VOC Sampling and Analysis
2.3. Statistical Analysis
2.4. Estimation of POCP and SOAP
2.4.1. Estimation of POCP
2.4.2. Estimation of SOAP
3. Results
3.1. Concentrations of VOCs during Dry Cleaning Process
3.2. Emission Factors for VOC Species
3.3. Photochemical Ozone Creation Potential (POCP) of VOCs
3.4. Second Organic Aerosol Potential (SOAP) of VOCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahu, L.K.; Saxena, P. High time and mass resolved PTR-TOF-MS measurements of VOCs at an urban site of India during winter: Role of anthropogenic, biomass burning, biogenic and photochemical sources. Atmos. Res. 2015, 164, 84–94. [Google Scholar] [CrossRef]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Xue, B.; Lv, Z.; Meng, Z.; Yang, X.; Xue, T.; Yu, Q.; He, K. Ground-level ozone pollution and its health impacts in China. Atmos. Environ. 2018, 173, 223–230. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Shin, H.-M.; McKone, T.E.; Bennett, D.H. Contribution of low vapor pressure-volatile organic compounds (LVP-VOCs) from consumer products to ozone formation in urban atmospheres. Atmos. Environ. 2015, 108, 98–106. [Google Scholar] [CrossRef]
- McDonald, B.C.; De Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Environmental Research (NIER). 2016 National Air Pollutants Emission. Available online: http://airemiss.nier.go.kr/module/statistics/materialStatistics.do?siteId=airemiss&id=airemiss_030200000000 (accessed on 15 April 2021).
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, M.E.; Derwent, R.G.; Wallington, T.J. Photochemical ozone creation potentials for volatile organic compounds: Rationalization and estimation. Atmos. Environ. 2017, 163, 128–137. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tan, X.; Fang, C. Analysis of NOx Pollution Characteristics in the Atmospheric Environment in Changchun City. Atmosphere 2020, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Tong, D.Q.; Lamsal, L.; Pan, L.; Ding, C.; Kim, H.; Lee, P.; Chai, T.; Pickering, K.E.; Stajnet, I. Long-term NOx trends over large cities in the United States during the great recession: Comparison of satellite retrievals, ground observations, and emission inventories. Atmos. Environ. 2015, 107, 70–84. [Google Scholar] [CrossRef]
- Best Practice Guidelines for Dry Cleaning: Issued to Support European Union Regulations 2012. Available online: https://www.epa.ie/pubs/reports/air/solvents/Best%20Practice%20Guidlines%20for%20Dry%20Cleaning%202013.pdf (accessed on 15 April 2021).
- USEPA Guideline Series: Control of Volatile Organic Compound Emissions from Large Petroleum Dry Cleaners. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/2000MHO3.TXT (accessed on 1 April 2021).
- National Institute of Environmental Research (NIER). A Study on the Application of Domestic VOC Emission Reduction in Advanced Countries; NIER: Incheon, Korea, 2007; pp. 26–30. [Google Scholar]
- Özden Üzmez, Ö.; Gaga, E.O.; Döğeroğlu, T. Development and field validation of a new diffusive sampler for determination of atmospheric volatile organic compounds. Atmos. Environ. 2015, 107, 174–186. [Google Scholar] [CrossRef]
- Lam, S.H.M.; Saunders, S.M.; Cheng, H.R.; Guo, H. Examination of regional ozone formation: POCPs for Western Australia and comparisons to other continents. Environ. Model. Softw. 2015, 74, 194–200. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Passant, N.R.; Pilling, M.J. Photochemical ozone creation potentials (POCPs) for different emission sources of organic compounds under European conditions estimated with a Master Chemical Mechanism. Atmos. Environ. 2007, 41, 2570–2579. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Utembe, S.R.; Shallcross, D.E.; Murrells, T.P.; Passant, N.P. Secondary organic aerosol formation from a large number of reactive man-made organic compounds. Sci. Total Environ. 2010, 408, 3374–3381. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Mass Generated | Compounds | Mass Generated | ||
|---|---|---|---|---|---|
| (1) | Decane | 2,035,713 | (19) | Acrolein | 15,349 |
| (2) | Nonane | 1,362,064 | (20) | 2-Methylpentane | 7512 |
| (3) | n-Undecane | 1,292,821 | (21) | Methyl ethyl ketone | 6191 |
| (4) | Hexane | 1,093,942 | (22) | Benzene | 4835 |
| (5) | 1,2-Dichloroethane | 827,240 | (23) | Ethylbenzene | 4733 |
| (6) | Methylene chloride | 709,959 | (24) | Tetrachloroethylene | 4446 |
| (7) | Acetone | 627,845 | (25) | Heptane | 3702 |
| (8) | Octane | 258,671 | (26) | Pentane | 3311 |
| (9) | n-Dodecane | 126,620 | (27) | 1-Butene | 2614 |
| (10) | Methylcyclopentane | 92,477 | (28) | Methyl methacrylate | 2241 |
| (11) | Cyclohexane | 86,727 | (29) | Styrene | 1959 |
| (12) | 2-Methylheptane | 37,636 | (30) | Methyl isobutyl ketone | 1712 |
| (13) | 3-Methylheptane | 29,288 | (31) | m, p-Xylene | 1649 |
| (14) | Toluene | 25,930 | (32) | Ethyl acetate | 748 |
| (15) | Isopentane | 24,410 | (33) | Butane | 743 |
| (16) | 3-Methylpentane | 16,969 | (34) | Vinyl Acetate | 726 |
| (17) | Tetra hydrofuran | 16,345 | (35) | Isoprene | 632 |
| (18) | Methylcyclohexane | 15,493 | (36) | Hexene | 250 |
| Compounds | POCP-Value | POCPweighted-emission | |
|---|---|---|---|
| (1) | Decane | 45 | 91.6 |
| (2) | Nonane | 45 | 61.3 |
| (3) | n-Undecane | 40 | 51.7 |
| (4) | Hexane | 40 | 43.8 |
| (5) | Octane | 50 | 12.9 |
| (6) | n-Dodecane | 45 | 5.7 |
| (7) | Methylcyclopentane | 50 | 4.6 |
| (8) | Cyclohexane | 25 | 2.2 |
| (9) | 2-Methylheptane | 50 | 1.9 |
| (10) | Toluene | 55 | 1.4 |
| Compounds | SOAP-Value | SOAPweighted-emission | |
|---|---|---|---|
| (1) | n-Undecane | 16.2 | 20.9 |
| (2) | Decane | 7 | 14.2 |
| (3) | n-Dodecane | 34.5 | 4.4 |
| (4) | Toluene | 100 | 2.6 |
| (5) | Nonane | 1.9 | 2.6 |
| (6) | Ethylbenzene | 111.6 | 0.5 |
| (7) | Benzene | 92.9 | 0.4 |
| (8) | Styrene | 212.3 | 0.4 |
| (9) | Octane | 0.8 | 0.2 |
| (10) | Acetone | 0.3 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, K.; Choi, Y.; Kim, D. Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality. Atmosphere 2021, 12, 637. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12050637
Lee H, Kim K, Choi Y, Kim D. Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality. Atmosphere. 2021; 12(5):637. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12050637
Chicago/Turabian StyleLee, Hyeonji, Kyunghoon Kim, Yelim Choi, and Daekeun Kim. 2021. "Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality" Atmosphere 12, no. 5: 637. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos12050637