Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Hospital Wastewater Sampling
2.3. Photocatalytic Materials
2.4. Photocatalytic Degradation Experiments
2.5. Analytical Procedures
2.5.1. Physicochemical Properties Analysis
2.5.2. SPE Extraction and UHPLC–LTQ/Orbitrap MS Analysis
3. Results and Discussion
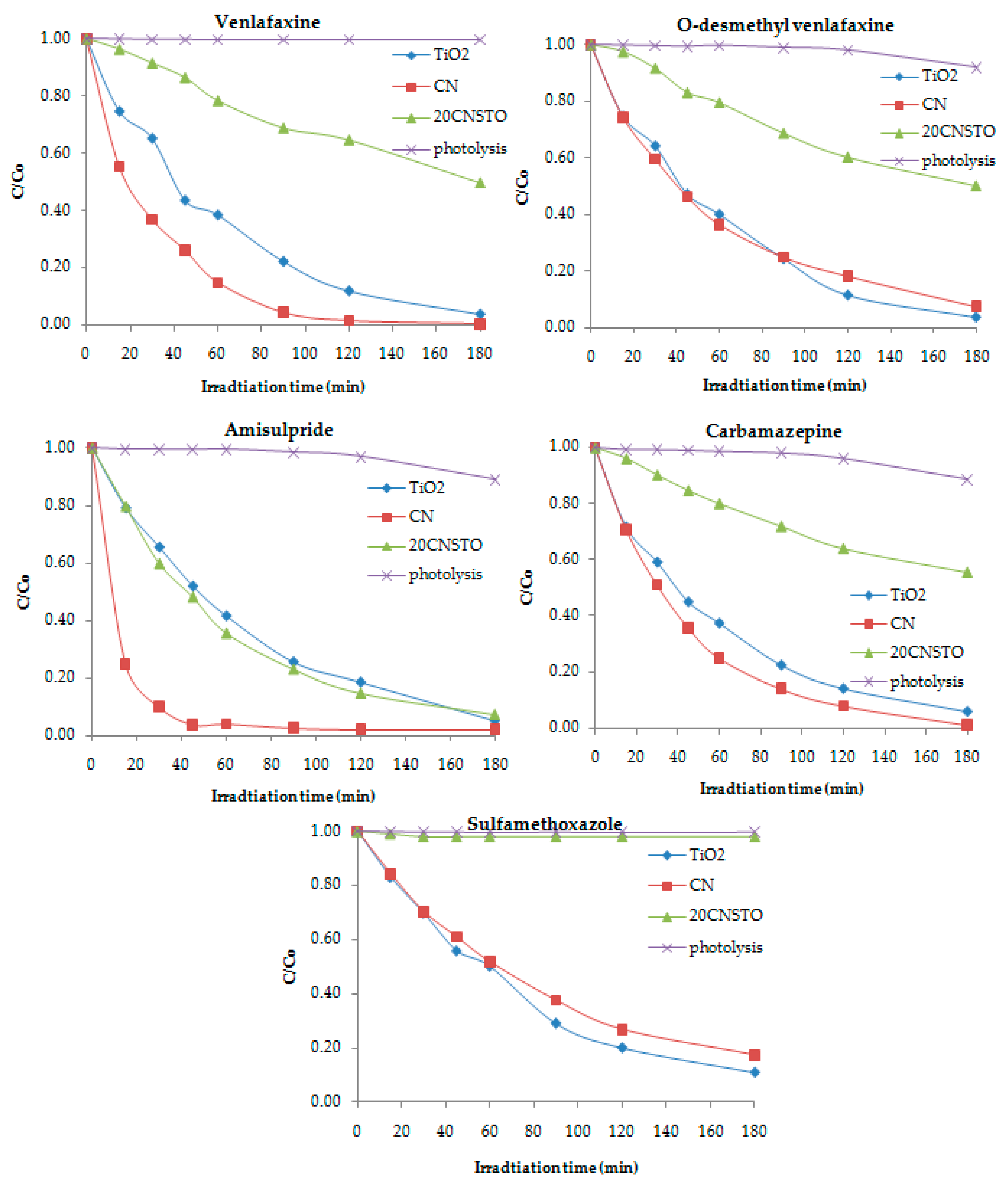
3.1. Degradation of Pharmaceuticals in the Hospital WWTP Effluent
3.2. Transformation Products/Metabolites Identification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Botero-Coy, A.M.; Moncayo-Lasso, A.; Hernández, F.; Torres-Palma, R.A. Effective elimination of fifteen relevant pharmaceuticals in hospital wastewater from Colombia by combination of a biological system with a sonochemical process. Sci. Total Environ. 2019, 670, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.S.; Da Silva, V.V.; Rosin, C.K.; Hainzenreder, L.; Arenzon, A.; Pizzolato, T.; Jank, L.; Feris, L.A. Determination of pharmaceutical compounds in hospital wastewater and their elimination by advanced oxidation processes. J. Environ. Sci. Health. Part A 2017, 53, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cuervo Lumbaque, E.; Cardoso, R.M.; Dallegrave, A.; dos Santos, L.O.; Ibáñez, M.; Hernández, F.; Sirtori, C. Pharmaceutical removal from different water matrixes by Fenton process at near-neutral pH: Doehlert design and transformation products identification by UHPLC-QTOF MS using a purpose-built database. J. Environ. Chem. Eng. 2018, 6, 3951–3961. [Google Scholar] [CrossRef]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kuhn, K.; Fauler, J.; Cuniberti, G. Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Comprehensive study of the antidiabetic drug metformin and its transformation product guanylurea in Greek wastewaters. Water Res. 2015, 70, 436–448. [Google Scholar] [CrossRef]
- Prieto-Rodriguez, L.; Oller, I.; Klamerth, N.; Aguera, A.; Rodrıguez, E.M.; Malato, S. Application of solar AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. 2013, 47, 1521–1528. [Google Scholar] [CrossRef]
- Lima Perini, J.A.; Tonetti, A.L.; Vidal, C.; Montagner, C.C.; Pupo Nogueira, R.F. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B Environ. 2018, 224, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Rioja, N.; Benguria, P.; Peñas, F.J.; Zorita, S. Competitive removal of pharmaceuticals from environmental waters by adsorption and photocatalytic degradation. Environ. Sci. Pollut. Res. 2014, 21, 11168–11177. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Development of SrTiO3 Photocatalysts with Visible Light Response Using Amino Acids as Dopant Sources for the Degradation of Organic Pollutants in Aqueous Systems. Catalysts 2018, 8, 528. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Zhang, M.; Chu, B.; Huang, W.-Y.; Fan, M.; Dong, L.; Li, B. The enhanced removal of toxic Cr(VI) in wastewater by synthetic TiO2/g-C3N4 microspheres/rGO photocatalyst under irradiation of visible light. Ind. Eng. Chem. Res. 2019, 58, 8979–8989. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Development of Fe-doped g-C3N4/graphitemediated peroxymonosulfate activation for degradation of aromatic pollutants via nonradical pathway. Sci. Total Environ. 2019, 675, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.-L.; Genga, Z.-Q.; Wang, W.; Yang, K.-C.; Wang, W.-P.; Han, C.-Q.; Yang, G.-H.; Vajtai, R.; Li, D.-W.; Ajayan, P.M. Recyclable three-dimensional Ag nanorod arrays decorated with O-g-C3N4 for highly sensitive SERS sensing of organic pollutants. J. Hazard. Mater. 2019, 379, 120823. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; An, X.; Huang, J. Preparation of a novel composite comprising biochar skeleton and “chrysanthemum” g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde. Appl. Surf. Sci. 2019, 487, 1262–1270. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Wark, M.; Mudring, A.-V. Low-temperature route to metal titanate perovskite nanoparticles for photocatalytic applications. Appl. Catal. B Environ. 2015, 178, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Rosy, A.; Kalpana, G. Reduced graphene oxide/strontium titanate heterostructured nanocomposite as sunlight driven photocatalyst for degradation of organic dye pollutants. Curr. Appl. Phys. 2018, 18, 1026–1033. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I.M.C. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Evgenidou, E.; Konstantinou, I.; Fytianos, K.; Poulios, I.; Albanis, T. Photocatalytic oxidation of methyl parathion over TiO2 and ZnO suspensions. Catal. Today 2007, 124, 156–162. [Google Scholar] [CrossRef]
- Lambropoulou, D.A.; Hernando, M.D.; Konstantinou, I.K.; Thurmanb, E.M.; Ferrer, I.; Albanis, T.A.; Fernandez-Alba, A.R. Identification of photocatalytic degradation products of bezafibrate in TiO2 aqueous suspensions by liquid and gas chromatography. J. Chromatogr. A 2008, 1183, 38–48. [Google Scholar] [CrossRef]
- Lambropoulou, D.A.; Konstantinou, I.K.; Albanis, T.A.; Fernandez-Alba, A.R. Photocatalytic degradation of the fungicide Fenhexamid in aqueous TiO2 suspensions: Identification of intermediates products and reaction pathways. Chemosphere 2011, 83, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Lambropoulou, D.A.; Evgenidou, E.; Saliverou, V.; Kosma, C.I.; Konstantinou, I.K. Degradation of venlafaxine using TiO2/UV process: Kinetic studies, RSM optimization, identification of transformation products and toxicity evaluation. J. Hazard. Mater. 2017, 323, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, D.R.; Konstantinou, I.K.; Mantzavinos, D.; Hela, D.; Papadaki, M. On the kinetics and mechanisms of photolytic/TiO2-photocatalytic degradation of substituted pyridines in aqueous solutions. Appl. Catal. B Environ. 2010, 95, 100–109. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. Optimization and Modeling of the Photocatalytic Degradation of the Insect Repellent DEET in Aqueous TiO2 Suspensions. CLEAN—Soil Air Water 2013, 41, 593–600. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. Photocatalytic treatment of metribuzin herbicide over TiO2aqueoussuspensions: Removal efficiency, identification of transformationproducts, reaction pathways and ecotoxicity evaluation. J. Photochem. Photobiol. A Chem. 2014, 294, 110–120. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. TiO2 photocatalysis of 2-isopropyl-3-methoxy pyrazine taste and odor compound in aqueous phase: Kinetics, degradation pathways and toxicity evaluation. Catal. Today 2015, 240, 22–29. [Google Scholar] [CrossRef]
- Stamatis, N.; Antonopoulou, M.; Hela, D.; Konstantinou, I.K. Photocatalytic degradation kinetics and mechanisms of antibacterial triclosan in aqueous TiO2 suspensions under simulated solar irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1145–1154. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Kazala, E.; Lambropoulou, D.A.; Kouras, A.; Kosma, C.I.; Albanis, T.A.; Poulios, I. Study of the decomposition and detoxification of the herbicidebentazon by heterogeneous photocatalysis: Kinetics, intermediatesand transformation pathways. Appl. Catal. B Environ. 2017, 200, 150–163. [Google Scholar] [CrossRef]
- Koltsakidou, Α.; Antonopoulou, M.; Εvgenidou, Ε.; Konstantinou, I.; Giannakas, A.E.; Papadaki, M.; Bikiaris, D.; Lambropoulou, D.A. Photocatalytical removal of fluorouracil using TiO2-P25 and N/S doped TiO2 catalysts: A kinetic and mechanistic study. Sci. Total Environ. 2017, 578, 257–267. [Google Scholar] [CrossRef]
- Moreira, N.; Sampaio, M.; Ribeiro, A.; Silva, C.; Faria, J.; Silva, A. Metal-free g-C3N4 photocatalysis of organic micropollutants in urban wastewater under visible light. Appl. Catal. B Environ. 2019, 248, 184–192. [Google Scholar] [CrossRef]
- Ding, N.; Chang, X.; Shi, N.; Yin, X.; Qi, F.; Sun, Y. Enhanced inactivation of antibiotic-resistant bacteria isolated from secondary effluents by g-C3N4 photocatalysis. Environ. Sci. Pollut. Res. 2019, 26, 18730–18738. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Memb. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Skeletal tailoring of two-dimensional π-conjugated polymer (g-C3N4) through sodium salt for solar-light driven photocatalysis. J. Photochem. Photobiol. A 2019, 373, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-structured CaTiO3 coupled with g-C3N4 as a heterojunction photocatalyst for organic pollutant degradation. Beilstein. J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; Varia, J.C.; Álvarez-Gallegos, A.; Ortiz-Hernández, M.L.; Peña-Camacho, J.L.; Silva-Martínez, S. Photoelectrocatalytic inactivation of fecal coliform bacteria in urban wastewater using nanoparticulated films of TiO2 and TiO2/Ag. Environ. Technol. 2017, 38, 606–614. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloy. Compd. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Li, X.; Liu, C.; Wang, H.; Huo, P.; Yan, Y.S. Molecularly imprinted Ag/Ag3VO4/g-C3N4 Z-scheme photocatalysts for enhanced preferential removal of tetracycline. J. Colloid Interface Sci. 2019, 552, 271–286. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Brar, S.K.; Daghrir, R.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y.; Drogui, P. Photocatalytic degradation of carbamazepine in wastewater by using a new class of whey-stabilized nanocrystalline TiO2 and ZnO. Sci. Total Environ. 2014, 485–486, 263–269. [Google Scholar] [CrossRef]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewaters: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.I.; Lambropoulou, D.A. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodriguez-Mozaz, S.; Barcelo, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Galána, M.J.; Anfruns, A.; Gonzalez-Olmos, R.; Rodríguez-Mozaz, S.; Comas, J. UV/H2O2 degradation of the antidepressants venlafaxine and O-desmethylvenlafaxine: Elucidation of their transformation pathwayand environmental fate. J. Hazard. Mater. 2016, 311, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, R. Identification of photodegradation product of amisulpride by ultra-high-pressure liquid chromatography–DAD/ESI-quadrupole time-of-flight-mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Williams, M.; Llorca, M.; Rodriguez-Mozaz, S.; Barceló, D.; Kookana, R.S. Photolysis of the antidepressants amisulpride and desipramine in wastewaters: Identification of transformation products formed and their fate. Sci. Total Environ. 2015, 530–531, 434–444. [Google Scholar] [CrossRef]
- Jelic, A.; Michael, I.; Achilleos, A.; Hapeshi, E.; Lambropoulou, D.; Perez, S.; Petrovic, M.; Fatta-Kassinos, D.; Barcelo, D. Transformation products and reaction pathways of carbamazepineduring photocatalytic and sonophotocatalytic treatment. J. Hazard. Mater. 2013, 263, 177–186. [Google Scholar] [CrossRef]
- Petrovic, M.; Barcelo, D. LC-MS for identifying photodegradation products of pharmaceuticals in the environment. Trends Anal. Chem. 2007, 26, 486–493. [Google Scholar] [CrossRef]
- Daniele, G.; Fieu, M.; Joachim, S.; Bado-Nilles, A.; Beaudouin, R.; Baudoin, P.; James-Casas, A.; Andres, S.; Bonnard, M.; Bonnard, I.; et al. Determination of carbamazepine and 12 degradation products in various compartments of an outdoor aquatic mesocosm by reliable analytical methods based on liquid chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. 2017, 24, 16893–16904. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Sakkas, V.A.; Albanis, T.A. Photocatalytic degradation of the herbicides propanil and molinate over aqueous TiO2 suspensions: Identification of intermediates and the reaction pathway. Appl. Catal. B Environ. 2001, 34, 227–239. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2002, 1310, 1–17. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Konstantinou, I.K.; Albanis, T.A. Photodegradation study of the antifouling booster biocide dichlofluanid in aqueous media by gas chromatographic techniques. J. Chromatogr. A 2001, 930, 135–144. [Google Scholar] [CrossRef]
- Czech, B.; Tyszczuk-Rotko, K. Caffeine hinders the decomposition of acetaminophen over TiO2-SiO2 nanocomposites containing carbon nanotubes irradiated by visible light. J. Photochem. Photobiol. A Chem. 2019, 376, 166–174. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Choi, Y.; Kim, S.; Lee, S.; Lee, S.; Choic, W.; Lee, J. Heterogeneous photocatalytic treatment of pharmaceuticalmicropollutants: Effects of wastewater effluent matrix and catalyst modifications. Appl. Catal. B Environ. 2014, 147, 8–16. [Google Scholar] [CrossRef]
- Mphela, R.K.; Msimanga, W.; Pete, K.Y.; Chiririwa, H.; Ochieng, A. Photocatalytic Degradation of Salicylic Acid and Reduction of Cr(VI) using TiO2. In Proceedings of the 5th International Conference on Advances in Engineering and Technology (ICAET’2016), New York, NY, USA, 8–9 June 2016; p. 30. [Google Scholar]
- Vilhunen, S.; Bosund, M.; Kaariainen, M.-L.; Cameron, D.; Sillanpaa, M. Atomic layer deposited TiO2 films in photodegradation of aqueous salicylic acid. Sep. Purif. Technol. 2009, 66, 130–134. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface Modification of TiO2 by Phosphate: Effect on Photocatalytic Activity and Mechanism Implication. J. Phys. Chem. C 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Photochemical transformation and wastewater fate and occurrence of omeprazole: HRMS for elucidation of transformation products and target and suspect screening analysis in wastewaters. Sci. Total Environ. 2017, 590–591, 592–601. [Google Scholar] [CrossRef]


| Parameter | February | March | May |
|---|---|---|---|
| Conductivity (μS/cm) | 289 | 373 | 344 |
| TDS (mg/L) | 417 | 398 | 357 |
| Temperature °C | 12.2 | 20.8 | 21.9 |
| Turbidity NTU | 9.4 | 11.5 | 6.8 |
| pH | 6.8 | 6.5 | 6.7 |
| COD (mg/L) | 15 | 16 | 13 |
| BOD5 (mg/L) | 9.6 | 9.6 | 7.9 |
| PO43− (mg/L) | 10.7 | 10.7 | 5.06 |
| NO3− (mg/L) | 134.1 | 120.3 | 80.4 |
| Pharmaceuticals | Ionization | Concentration (ng/L) | ||
|---|---|---|---|---|
| Mode | February | March | May | |
| Analgesic/Anti-inflammatory | ||||
| Salicylic acid | − | 236.4 | 239.9 | <LOQ |
| Diclofenac | − | 181.1 | 181.0 | 79.2 |
| Lipid regulator | ||||
| Fenofibrate | + | n.d. | n.d. | 128.9 |
| Antibiotic | ||||
| Trimethoprim | + | n.d. | 97.0 | 21.5 |
| Sulfamethoxazole | + | n.d. | 157.9 | 349.8 |
| Antidepressant | ||||
| Venlafaxine | + | 434.2 | 412.5 | 391.2 |
| O-desmethyl venlafaxine | + | 691.4 | 1871.0 | 748.5 |
| Mirtazapine | + | n.d. | n.d. | 43.2 |
| Bupropion | + | n.d. | n.d. | 15.8 |
| Paroxetine | + | 32.4 | n.d. | n.d. |
| Fluoxetine | + | n.d. | 36.7 | n.d. |
| Sertraline | + | n.d. | 68.4 | n.d. |
| Citalopram | + | 102.4 | 55.9 | 39.4 |
| Amitryptiline | + | 23.0 | n.d. | n.d. |
| Fluvoxamine | + | n.d. | n.d. | 8.5 |
| Antiepiliptic | ||||
| Carbamazepine | + | 388.2 | 266.7 | 242.0 |
| Antipsychotic | ||||
| Haloperidol | + | 42.0 | n.d. | n.d. |
| Clozapine | + | 67.8 | n.d. | n.d. |
| Amisulpride | + | 102.0 | 929.4 | 505.5 |
| Compound | February | March | May | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k (min−1) | t1/2 (min) | R2 | k (min−1) | t1/2 (min) | R2 | k (min−1) | t1/2 (min) | R2 | |
| VNX | 0.0124 | 55.90 | 0.9971 | 0.0104 | 66.50 | 0.9918 | 0.0176 | 39.38 | 0.9956 |
| ODV | 0.0131 | 52.91 | 0.9971 | 0.0097 | 71.46 | 0.9902 | 0.0177 | 39.16 | 0.9900 |
| AMS | 0.0046 | 150.68 | 0.9922 | 0.0108 | 64.80 | 0.9915 | 0.0155 | 44.52 | 0.9900 |
| SMX | - | - | - | 0.0022 | 315.07 | 0.9914 | 0.0128 | 54.15 | 0.9937 |
| CBZ | 0.0071 | 97.63 | 0.9908 | 0.0054 | 128.36 | 0.9933 | 0.0161 | 43.05 | 0.9962 |
| Compound | TiO2 | CN | 20CNSTO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k (min−1) | t1/2 (min) | R2 | k (min−1) | t1/2 (min) | R2 | k (min−1) | t1/2 (min) | R2 | |
| VNX | 0.0176 | 39.38 | 0.9956 | 0.0327 | 21.2 | 0.9909 | 0.0038 | 182.41 | 0.9913 |
| ODV | 0.0177 | 39.16 | 0.9900 | 0.0148 | 46.83 | 0.9903 | 0.0040 | 173.29 | 0.9902 |
| AMS | 0.0155 | 44.52 | 0.9900 | 0.0308 | 22.50 | 0.9970 | 0.0153 | 45.30 | 0.9901 |
| SMX | 0.0128 | 54.15 | 0.9937 | 0.0103 | 67.30 | 0.9902 | - | - | - |
| CBZ | 0.0161 | 43.05 | 0.9962 | 0.0232 | 29.88 | 0.9905 | 0.0035 | 198.04 | 0.9901 |
| Parameter | Hospital Wastewater before PC | Hospital Wastewater after PC-TiO2-P25 | Hospital Wastewater after PC-CN | Hospital Wastewater after PC-20CNSTO |
|---|---|---|---|---|
| Conductivity (μS/cm) | 344 | 403 | 393 | 407 |
| TDS (mg/L) | 357 | 371 | 372 | 373 |
| pH | 6.7 | 6.7 | 7 | 6.8 |
| COD (mg/L) | 13 | <10 | <10 | <10 |
| PO43− (mg/L) | 5.06 | 3.38 | 4.22 | 3.8 |
| NO3− (mg/L) | 80.4 | 158.7 | 165.1 | 120.4 |
| TPs/ Metabolites | Rt (min) | Elemental Formula [M + H]+ | Accurate Mass | Error (ppm) | DBE | Catalyst Occurrence | |
|---|---|---|---|---|---|---|---|
| Theor. | Exper. | ||||||
| VNX | 3.14 | C17H28NO2 | 278.2115 | 278.2126 | 3.934 | 4.5 | TiO2, CN, 20CNSTO |
| VNX-TP1 | 3.02 | C16H24NO2 | 262.1802 | 262.1805 | 1.238 | 5.5 | TiO2, CN, 20CNSTO |
| VNX-TP2 | 2.15 | C16H26NO3 | 280.1903 | 280.1912 | 1.784 | 4.5 | TiO2, CN |
| VNX-TP3a | 2.36 | C15H24NO2 | 250.1802 | 250.1808 | 2.776 | 4.5 | TiO2, CN, 20CNSTO |
| VNX-TP3b | 2.88 | C15H24NO2 | 250.1802 | 250.1811 | 3.575 | 4.5 | TiO2, CN, 20CNSTO |
| ODV | 2.72 | C16H26NO2 | 264.1958 | 264.1967 | 3.575 | 4.5 | TiO2, CN, 20CNSTO |
| ODV-ΤP1 | 2.17 | C16H24NO | 246.1852 | 246.1854 | 0.646 | 5.5 | TiO2, CN, 20CNSTO |
| AMS | 2.53 | C17H28N3O4S | 370.1795 | 370.1805 | 2.719 | 5.5 | TiO2, CN, 20CNSTO |
| AMS-TP1 | 2.69 | C17H28N3O5S | 386.1744 | 386.1747 | 0.678 | 5.5 | TiO2, CN, 20CNSTO |
| CBZ | 3.60 | C15H13N2O | 237.1022 | 237.1028 | 2.279 | 10.5 | TiO2, CN, 20CNSTO |
| CBZ-ΤP1 | 3.20 | C15H13N2O2 | 253.0972 | 253.0976 | 1.682 | 10.5 | TiO2, CN, 20CNSTO |
| CBZ-ΤP2 | 3.28 | C15H13N2O2 | 253.0972 | 253.0976 | 1.682 | 10.5 | TiO2, CN, 20CNSTO |
| CBZ-ΤP3 | 3.40 | C15H13N2O2 | 253.0972 | 253.0974 | 0.971 | 10.5 | TiO2, CN, 20CNSTO |
| CBZ-ΤP4 | 3.55 | C15H13N2O2 | 253.0972 | 253.0982 | 4.211 | 10.5 | TiO2, CN |
| CBZ-ΤP5 | 3.22 | C15H15N2O3 | 271.1077 | 271.1083 | 2.955 | 9.5 | TiO2, CN, 20CNSTO |
| SMX | 2.77 | C10H12N3O3S | 254.0594 | 254.0600 | 0.612 | 6.5 | TiO2, CN, 20CNSTO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstas, P.-S.; Kosma, C.; Konstantinou, I.; Albanis, T. Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration. Water 2019, 11, 2165. https://0-doi-org.brum.beds.ac.uk/10.3390/w11102165
Konstas P-S, Kosma C, Konstantinou I, Albanis T. Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration. Water. 2019; 11(10):2165. https://0-doi-org.brum.beds.ac.uk/10.3390/w11102165
Chicago/Turabian StyleKonstas, Panagiotis-Spyridon, Christina Kosma, Ioannis Konstantinou, and Triantafyllos Albanis. 2019. "Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration" Water 11, no. 10: 2165. https://0-doi-org.brum.beds.ac.uk/10.3390/w11102165




