Zooplankton Size as a Factor Determining the Food Selectivity of Roach (Rutilus Rutilus) in Water Basin Outlets
Abstract
:1. Introduction
2. Methods
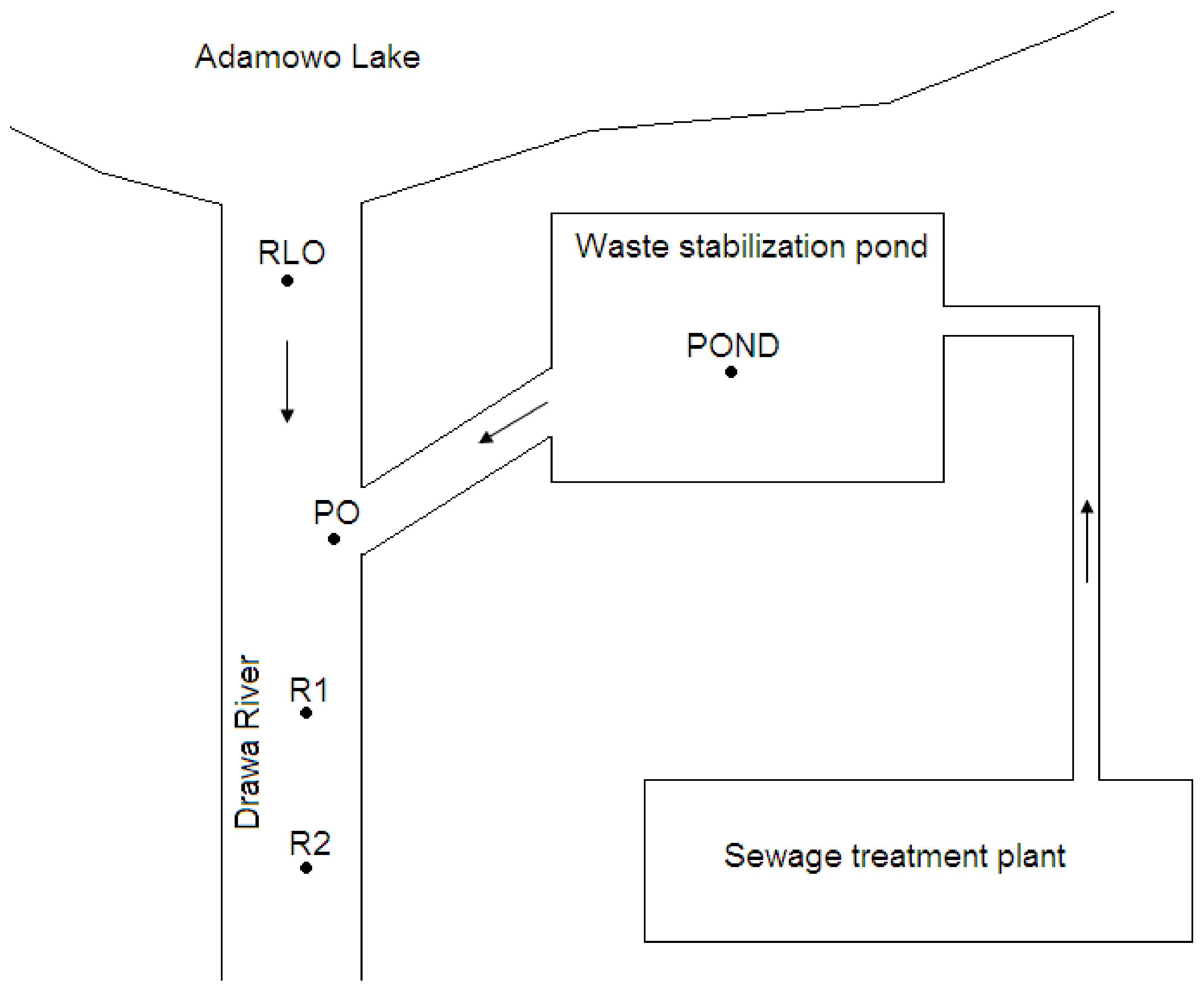
2.1. Study Area
2.2. Sampling Methods
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gliwicz, Z.M. A lunar cycle in zooplankton. Ecology 1986, 67, 883–897. [Google Scholar] [CrossRef]
- Christoffersen, K.; Riemann, B.; Klysner, A.; Søndergaard, M. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnol. Oceanogr. 1993, 38, 561–573. [Google Scholar] [CrossRef]
- Wissel, B.; Boeing, W.J.; Ramcharan, C.W. Effects of water color on predation regimes and zooplankton assemblages in freshwater lakes. Limnol. Oceanogr. 1998, 48, 1965–1976. [Google Scholar] [CrossRef]
- Hoffman, J.C.; Smith, M.E.; Lehman, J.T. Perch or plankton: Top-down control of Daphnia by yellow perch (Perca flavescens) or Bythotrephes cederstroemi in an inland lake? Freshw. Biol. 2001, 46, 759–775. [Google Scholar] [CrossRef]
- Jack, J.D.; Thorp, J.H. Impacts of fish predation on an Ohio River zooplankton community. J. Plankton Res. 2002, 24, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Czerniawski, R.; Domagała, J. Reduction of zooplankton communities in small lake outlets in relation to abiotic and biotic factors. Oceanol. Hydrobiol. Stud. 2013, 42, 123–131. [Google Scholar] [CrossRef]
- Czerniawski, R.; Sługocki, Ł.; Kowalska-Góralska, M. Diurnal changes of zooplankton community reduction rate at lake outlets and related environmental factors. PLoS ONE 2016, 11, e0158837. [Google Scholar] [CrossRef]
- Walks, D.J.; Cyr, H. Movement of plankton through lake-stream systems. Freshw. Biol. 2004, 49, 745–759. [Google Scholar] [CrossRef]
- Chang, K.H.; Doi, H.; Imai, H.; Gunji, F.; Nakano, S.I. Longitudinal changes in zooplankton distribution below a reservoir outfall with reference to river planktivory. Limnology 2008, 9, 125–133. [Google Scholar] [CrossRef]
- Mehner, T. Influence of spring warming on the predation rate of underyearling fish on daphnia—A deterministic simulation approach. Freshw. Biol. 2000, 45, 253–263. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.M.; Nagengast, B. The influence of the spatial structure of hydromacrophytes and differentiating habitat on the structure of rotifer and cladoceran communities. Hydrobiologia 2006, 559, 203–212. [Google Scholar] [CrossRef]
- Estlander, S.; Nurminen, L.; Olin, M.; Vinni, M.; Horppila, J. Seasonal fluctuations in macrophyte cover and water transparency of four brown-water lakes: Implications for crustacean zooplankton in littoral and pelagic habitats. Hydrobiologia 2009, 620, 109–120. [Google Scholar] [CrossRef]
- Kamarainen, A.M.; Rowland, F.E.; Biggs, R.; Carpenter, S.R. Zooplankton and the total phosphorus-chlorophyll a relationship: Hierarchical bayesian analysis of measurement error. Can. J. Fish. Aquat. Sci. 2008, 65, 2644–2655. [Google Scholar] [CrossRef]
- Gołdyn, R.; Kowalczewska-Madura, K. Interactions between phytoplankton and zooplankton in the hypertrophic swarzędzkie Lake in western Poland. J. Plankton Res. 2008, 30, 33–42. [Google Scholar]
- Pourriot, R.; Rougier, C.; Miquelis, A. Origin and development of river zooplankton: Example of the Marne. Hydrobiologia 1997, 345, 143–148. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J.; Kruk, M. Effects of contrasting land use on free-swimming rotifer communities of streams in Masurian Lake District, Poland. Hydrobiologia 1998, 387/388, 241–249. [Google Scholar] [CrossRef]
- Radwan, S. Wrotki (Rotifera); Wydawnictwo Uniwersytetu Łódzkiego: Łódź, Polska, 2004; pp. 1–447. [Google Scholar]
- Rybak, J.I.; Błędzki, L.A. Planktonic Crustaceans of Freshwaters; Wydawnictwo Uniwersytetu Warszawskiego: Warszawa, Poland, 2010; pp. 1–366. [Google Scholar]
- Czerniawski, R.; Domagała, J. Small dams profoundly alter the spatial and temporal composition of zooplankton communities in running waters. Int. Rev. Hydrobiol. 2014, 99, 300–311. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modem Applied Statistics with S. Fourth Edition; Springer: New York, USA, 2002; pp. 1–487. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing—R Foundation for Statistical Computing; Vienna, Austria. 2018. Available online: https://www.R-project.org (accessed on 20 December 2018).
- Czerniawski, R.; Domagała, J. Zooplankton communities of two lake outlets in relation to abiotic factors. Open Life Sci. 2010, 5, 240–255. [Google Scholar] [CrossRef]
- Czerniawski, R.; Domagała, J. Similarities in zooplankton community between river drawa and its two tributaries (Polish part of River Odra). Hydrobiologia 2010, 638, 137–149. [Google Scholar] [CrossRef]
- Bone, Q.; Moore, R. Biology of Fishes; Taylor and Francis: New York, NY, USA, 2008; pp. 1–497. [Google Scholar]
- Akopian, M.; Garnier, J.; Pourriot, R. A large reservoir as a source of zooplankton for the river: Structure of the populations and influence of fish predation. J. Plankton Res. 1999, 21, 285–297. [Google Scholar] [CrossRef]
- Meeren, T.; Olsen, E.R.; Hamre, K.; Fyhn, J.H. Biochemical composition of copepods for evaluation of feed production of juvenile marine fish. Aquaculture 2008, 274, 375–379. [Google Scholar] [CrossRef]



| Date | Fish Age | Length (cm) | Mass (g) | Condition Factor |
|---|---|---|---|---|
| 9th August | 6+ (n = 2) | 13.70 ± 0.57 | 35.05 ± 5.16 | 1.36 ± 0.03 |
| 5+ (n = 10) | 10.88 ±1.05 | 17.25 ± 4.92 | 1.31 ± 0.08 | |
| 4+ (n = 7) | 10.31 ± 0.39 | 13.80 ± 1.91 | 1.25 ± 0.11 | |
| 3+ (n = 11) | 8.69 ± 0.34 | 8.09 ± 1.58 | 1.22 ± 0.13 | |
| 16th August | 6+ (n = 4) | 12.58 ± 0.62 | 23.90 ± 3.63 | 1.20 ± 0.10 |
| 5+ (n = 6) | 10.57 ± 1.05 | 14.78 ± 3.55 | 1.24 ± 0.10 | |
| 4+ (n = 13) | 9.14 ± 0.82 | 9.30 ± 2.99 | 1.17 ± 0.11 | |
| 3+ (n = 5) | 7.42 ± 0.22 | 4.72 ± 0.69 | 1.15 ± 0.08 | |
| 2+ (n = 2) | 6.20 ± 0.14 | 2.55 ± 0.07 | 1.07 ± 0.10 | |
| 20th August | 7+ (n = 1) | 14.30 | 39.40 | 1.35 |
| 6+ (n = 7) | 12.37 ± 0.76 | 24.63 ± 3.93 | 1.29 ± 0.09 | |
| 5+ (n = 7) | 11.03 ± 0.73 | 17.30 ± 3.96 | 1.27 ± 0.07 | |
| 4+ (n = 5) | 9.40 ± 0.72 | 10.74 ± 2.44 | 1.28 ± 0.05 | |
| 3+ (n = 9) | 7.42 ± 0.36 | 5.19 ± 0.77 | 1.26 ± 0.07 | |
| 2+ (n = 1) | 5.60 | 2.20 | 1.25 | |
| 4th September | 6+ (n = 9) | 12.16 ± 0.30 | 23.14 ± 1.90 | 1.29 ± 0.04 |
| 5+ (n = 9) | 11.38 ± 0.31 | 18.88 ± 1.71 | 1.28 ± 0.05 | |
| 4+ (n = 3) | 8.47 ± 0.46 | 7.50 ± 1.68 | 1.22 ± 0.09 | |
| 3+ (n = 9) | 7.49 ± 0.33 | 5.31 ± 0.94 | 1.25 ± 0.10 |
| Zooplankton Group | RLO | POND | PO | R1 | R2 |
|---|---|---|---|---|---|
| Asplanchna | 17.4 ± 18.2 a | 4.7 ± 8.9 ab | 2.5 ± 5.0 b | 12.4 ± 11.2 ab | 9.6 ± 9.6 ab |
| Benthic rotifers | 18.9 ± 11.6 a | 4.3 ± 6.8 a | 2.4 ± 1.1 a | 23.3 ± 22.0 a | 24.5 ± 17.9 a |
| Planktonic rotifers | 351.5 ± 216.7 a | 37.7 ± 30.3 ab | 24.9 ± 21.2 b | 313.6 ± 178.8 ab | 325.4 ± 202.5 ab |
| Small cladocerans | 38.6 ± 16.8 ab | 54.2 ± 51.8 ab | 39.7 ± 45.4 ab | 46.8 ± 32.8 ab | 28.3 ± 15.8 ab |
| Large cladocerans | 6.0 ± 3.9 ab | 0.6 ± 0.7 ab | - | 2.9 ± 2.1 ab | 1.0 ± 0.5 ab |
| Daphnia pulex | - | 122.9 ± 62.5 a | 84.4 ± 57.5 a | 9.1 ± 9.6 b | 0.8 ± 1.0 b |
| Nauplii Cyclopoida | 57.5 ± 59.3 a | 7.7 ± 6.5 ab | 4.5 ± 3.1 b | 61.4 ± 73.8 ab | 50.5 ± 65.3 ab |
| Cyclopoida | 21.2 ± 10.2 a | 6.9 ± 2.9 b | 3.9 ± 2.4 b | 20.6 ± 9.3 a | 8.5 ± 4.6 ab |
| Fixed Effects | Estimate | Std. Error | z Value | p |
|---|---|---|---|---|
| (Intercept) | 0.803 | 0.426 | 1.883 | 0.060 |
| R1 vs PO | 1.492 | 0.492 | 3.035 | 0.002 |
| R2 vs PO | 1.248 | 0.500 | 2.496 | 0.013 |
| Benthic rotifers | −0.311 | 0.602 | −0.516 | 0.606 |
| Cyclopoida | 0.336 | 0.538 | 0.625 | 0.532 |
| Daphnia pulex | 1.825 | 0.581 | 3.142 | 0.002 |
| Large cladocerans | −21.717 | 42.375 | −0.512 | 0.608 |
| Nauplii Cyclopoida | 0.251 | 0.538 | 0.466 | 0.641 |
| Planktonic rotifers | 1.167 | 0.594 | 1.965 | 0.049 |
| Small cladocerans | 1.678 | 0.502 | 3.344 | 0.001 |
| RLO | 0.001 | 0.001 | 1.209 | 0.227 |
| POND | 0.013 | 0.003 | 4.384 | <0.001 |
| R1 vs PO: Benthic rotifers | 0.951 | 0.714 | 1.333 | 0.182 |
| R2 vs PO: Benthic rotifers | 1.273 | 0.719 | 1.769 | 0.077 |
| R1 vs PO: Cyclopoida | 0.240 | 0.663 | 0.362 | 0.717 |
| R2 vs PO: Cyclopoida | −0.400 | 0.682 | −0.586 | 0.558 |
| R1 vs PO: Daphnia pulex | −3.903 | 0.635 | −6.149 | <0.001 |
| R2 vs PO: Daphnia pulex | −6.017 | 0.850 | −7.075 | <0.001 |
| R1 vs PO: Large cladocerans | 20.295 | 42.376 | 0.479 | 0.632 |
| R2 vs PO: Large cladocerans | 18.908 | 42.377 | 0.446 | 0.655 |
| R1 vs PO: Nauplii Cyclopoida | 1.137 | 0.656 | 1.733 | 0.083 |
| R2 vs PO: Nauplii Cyclopoida | 1.148 | 0.663 | 1.730 | 0.084 |
| R1 vs PO: Planktonic rotifers | 1.282 | 0.614 | 2.087 | 0.037 |
| R2 vs PO: Planktonic rotifers | 1.527 | 0.621 | 2.458 | 0.014 |
| R1 vs PO: Small cladocerans | −0.993 | 0.614 | −1.619 | 0.106 |
| R2 vs PO: Small cladocerans | −1.173 | 0.624 | −1.879 | 0.060 |
| Fish age | 2+ | 3+ | 4+ | 5+ | 6+ | 7+ | |
| n | 3 | 34 | 28 | 32 | 22 | 1 | |
| Zooplankton group | Asplanchna | - | 0.03 ± 0.10 a | 0.02 ± 0.09 a | 0.01 ± 0.04 a | 0.01 ± 0.03 a | - |
| Benthic rotifers | 0.88 ± 1.52 a | 0.10 ± 0.31 a | 0.02 ± 0.06 a | - | - | - | |
| Pelagic rotifers | 1.02 ± 0.91 a | 0.23 ± 0.47 a | 0.03 ± 0.10 a | 0.02 ± 0.08 a | - | - | |
| Small cladocerans | 2.50 ± 0.22 a | 0.40 ± 0.83 ab | 0.16 ± 0.35 ab | 0.05 ± 0.12 b | 0.04 ± 0.07 b | 0.25 ab | |
| Large cladocerans | 0.88 ± 1.52 a | 0.26 ± 0.47 a | 0.15 ± 0.23 a | 0.10 ± 0.16 a | 0.09 ± 0.12 a | 0.08 a | |
| Daphnia pulex | 90.96 ± 7.01 a | 98.11 ± 2.13 a | 99.16 ± 1.13 ab | 99.65 ± 0.32 ab | 99.74 ± 0.27 b | 99.58 ab | |
| Nauplii | 3.32 ± 3.29 a | 0.39 ± 0.62 ab | 0.18 ± 0.40 ab | 0.02 ± 0.07 b | 0.01 ± 0.03 b | - | |
| Cyclopoida | 0.44 ± 0.76 a | 0.47 ± 0.80 a | 0.27 ± 0.52 a | 0.15 ± 0.20 a | 0.12 ± 0.20 a | - | |
| Site RLO | n | r | p |
|---|---|---|---|
| Ab. Asplanchna vs. Stom. Asplanchna | 4 | 0.74 | 0.262 |
| Ab. Benthic rotifers vs. Stom. Benthic rotifers | 4 | −0.74 | 0.262 |
| Ab. Planktonic rotifers vs. Stom. Pelagic rotifers | 4 | 0.32 | 0.683 |
| Ab. Small cladocerans vs. Stom. Small cladocerans | 4 | 0.80 | 0.200 |
| Ab. Large cladocerans vs. Stom. Large cladocerans | 4 | 0.99 | 0.001 |
| Ab. Daphnia pulex vs. Stom. Daphnia pulex | 1 | - | - |
| Ab. Nauplii Cyclopoida vs. Stom. Nauplii | 4 | 0.63 | 0.367 |
| Ab. Cyclopoida vs. Stom. Cyclopoida | 4 | 0.80 | 0.200 |
| Site PO | |||
| Ab. Asplanchna vs. Stom. Asplanchna | 1 | - | - |
| Ab. Benthic rotifers vs. Stom. Benthic rotifers | 4 | 0.94 | 0.051 |
| Ab. Planktonic rotifers vs. Stom. Pelagic rotifers | 4 | 0.32 | 0.684 |
| Ab. Small cladocerans vs. Stom. Small cladocerans | 4 | 0.80 | 0.200 |
| Ab. Large cladocerans vs. Stom. Large cladocerans | 0 | - | - |
| Ab. Daphnia pulex vs. Stom. Daphnia pulex | 4 | 0.80 | 0.200 |
| Ab. Nauplii Cyclopoida vs. Stom. Nauplii | 4 | 0.83 | 0.167 |
| Ab. Cyclopoida vs. Stom. Cyclopoida | 4 | 0.60 | 0.400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerniawski, R.; Krepski, T. Zooplankton Size as a Factor Determining the Food Selectivity of Roach (Rutilus Rutilus) in Water Basin Outlets. Water 2019, 11, 1281. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061281
Czerniawski R, Krepski T. Zooplankton Size as a Factor Determining the Food Selectivity of Roach (Rutilus Rutilus) in Water Basin Outlets. Water. 2019; 11(6):1281. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061281
Chicago/Turabian StyleCzerniawski, Robert, and Tomasz Krepski. 2019. "Zooplankton Size as a Factor Determining the Food Selectivity of Roach (Rutilus Rutilus) in Water Basin Outlets" Water 11, no. 6: 1281. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061281





