Evaluation of Mercury Transformation and Benthic Organisms Uptake in a Creek Sediment of Pearl River Estuary, China
Abstract
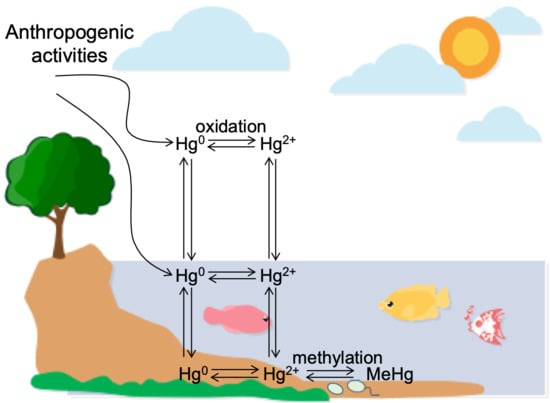
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sediment Sample Collection
2.3. Determination of the Total Mercury in Sediment Samples
2.4. Determination of the Simultaneously Extracted Mercury in Sediment Samples
2.5. Determination of the Methylmercury Content in the Sediment Samples
2.6. Determination of Acid-Volatile Sulfide Content in Sediment Samples
2.7. Determination of Sulfate-Reducing Bacteria Amount in Sediment Samples
2.8. Determination of Redox Potential and Organic Carbon in the Sediment Samples
2.9. Determination of Mercury Accumulated in Benthic Organisms
3. Results and Discussions
3.1. Mercury Measurement and Speciation in Sediment Sites
3.2. Mercury Speciation in the Sediments
3.3. Reduced Benthos Biodiversity Due to Uptake of Methylmercury
3.4. Correlation Analysis of Methylmercury Formation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Eto, K. Pathology of Minamata disease. Toxicol. Pathol. 1997, 25, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Ekino, S.; Susa, M.; Ninomiya, T.; Imamura, K.; Kitamura, T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J. Neurol. Sci. 2007, 262, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, G.; Rui, Z.; Alshawabkeh, A.N. Demonstration of a feasible energy-water-environment nexus: Waste sulfur dioxide for water treatment. Appl. Energy 2019, 250, 1011–1022. [Google Scholar] [CrossRef]
- Martín, J.A.R.; Nanos, N. Soil as an archive of coal-fired power plant mercury deposition. J. Hazard. Mater. 2016, 308, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Lu, Z.; Levin, L.; ter Schure, A.F.; Sunderland, E.M. Historical releases of mercury to air, land, and water from coal combustion. Sci. Total Environ. 2018, 615, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Toimela, T.; Tähti, H. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch. Toxicol. 2004, 78, 565–574. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Mitchener, M. Life-term effects of mercury, methyl mercury, and nine other trace metals on mice. J. Nutr. 1975, 105, 452–458. [Google Scholar] [CrossRef]
- Magour, S.; Mäser, H.; Greim, H. The Effect of Mercury Chloride and Methyl Mercury on Brain Microsomal Na+-K+-ATPase after Partial Delipidisation with Lubrol®. Pharmacol. Toxicol. 1987, 60, 184–186. [Google Scholar] [CrossRef]
- Knap, A.; Dewailly, É.; Furgal, C.; Galvin, J.; Baden, D.; Bowen, R.E.; Depledge, M.; Duguay, L.; Fleming, L.E.; Ford, T.; et al. Indicators of ocean health and human health: Developing a research and monitoring framework. Environ. Health Perspect. 2002, 110, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.C.; Folt, C.L.; Chen, C.Y.; Klaue, B.; Blum, J.D. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA 2002, 99, 4419–4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M. Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut. 1995, 80, 915–921. [Google Scholar] [CrossRef]
- Chen, C.Y.; Borsuk, M.E.; Bugge, D.M.; Hollweg, T.; Balcom, P.H.; Ward, D.M.; Williams, J.; Mason, R.P. Benthic and pelagic pathways of methylmercury bioaccumulation in estuarine food webs of the northeast United States. PLoS ONE 2014, 9, e89305. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.F.; Bugge, D.; Jackson, B.P.; Chen, C.Y. Pathways of CH3Hg and Hg ingestion in benthic organisms: An enriched isotope approach. Environ. Sci. Technol. 2014, 48, 5058–5065. [Google Scholar] [CrossRef] [PubMed]
- Hornung, H.; Krumgalz, B.S.; Cohen, Y. Mercury pollution in sediments, benthic organisms and inshore fishes of Haifa Bay, Israel. Mar. Environ. Res. 1984, 12, 191–208. [Google Scholar] [CrossRef]
- Pickhardt, P.C.; Fisher, N.S. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ. Sci. Technol. 2007, 41, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Carmichael, N.; Cavanagh, J.B. Ultrastructural changes in the nervous system of rabbits poisoned with methyl mercury. Toxicol. Appl. Pharmacol. 1977, 39, 249–261. [Google Scholar] [CrossRef]
- Clarkson, T.W. Metal toxicity in the central nervous system. Environ. Health Perspect. 1987, 75, 59–64. [Google Scholar] [CrossRef]
- Rodier, P.M. Vulnerable periods and processes during central nervous system development. Environ. Health Perspect. 1994, 102, 121–124. [Google Scholar]
- Howie, M.G.; Jackson, A.K.; Cristol, D.A. Spatial extent of mercury contamination in birds and their prey on the floodplain of a contaminated river. Sci. Total Environ. 2018, 630, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Santschi, P.H.; Yeager, K.M.; Schwehr, K.A.; Schindler, K.J. Estimates of recovery of the Penobscot River and estuarine system from mercury contamination in the 1960’s. Sci. Total Environ. 2017, 596, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.G.; Bouchet, S.; Guédron, S.; Amouroux, D.; Dominik, J.; Zopfi, J. High methylmercury production under ferruginous conditions in sediments impacted by sewage treatment plant discharges. Water Res. 2015, 80, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Elias, D.A.; Kucken, A.M.; Brown, S.D.; Palumbo, A.V.; Schadt, C.W.; Wall, J.D. Sulfate-reducing bacterium Desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Appl. Environ. Microbiol. 2011, 77, 3938–3951. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Podar, M.; Bullock, A.L.; Graham, A.M.; Brown, S.D.; Somenahally, A.C.; Johs, A.; Hurt, R.A., Jr.; Bailey, K.L.; Elias, D.A. Mercury methylation by novel microorganisms from new environments. Environ. Sci. Technol. 2013, 47, 11810–11820. [Google Scholar] [CrossRef] [PubMed]
- Bloom, N.S.; Lasorsa, B.K. Changes in mercury speciation and the release of methylmercury as a result of marine sediment dredging activities. Sci. Total Environ. 1999, 237–238, 379–385. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Gobas, F.A.; Branfireun, B.A.; Heyes, A. Environmental controls on the speciation and distribution of mercury in coastal sediments. Mar. Chem. 2006, 102, 111–123. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamation-affected rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef]
- Zhao, G.; Ye, S.; Yuan, H.; Ding, X.; Wang, J. Surface sediment properties and heavy metal pollution assessment in the Pearl River Estuary, China. Environ. Sci. Pollut. Res. 2017, 24, 2966–2979. [Google Scholar] [CrossRef]
- Li, X.; Wai, O.W.; Li, Y.S.; Coles, B.J.; Ramsey, M.H.; Thornton, I. Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Appl. Geochem. 2000, 15, 567–581. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Cui, B.; Zhang, K.; Wang, Q.; Liu, X.; Gao, H.; Huang, L. Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environ. Pollut. 2011, 159, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Li, T. The Water Quality and Water Environment Capacity of Main Rivers in Foshan, Guangdong Province. Master’s Thesis, Sun Yat-Sen University, Guangzhou, China, 2005. (In Chinese). [Google Scholar]
- Hatch, W.R.; Ott, W.L. Determination of submicrogram quantities of mercury by atomic absorption spectrophotometry. Anal. Chem. 1968, 40, 2085–2087. [Google Scholar] [CrossRef]
- Tseng, C.M.; De Diego, A.; Martin, F.M.; Amouroux, D.; Donard, O.F. Rapid determination of inorganic mercury and methylmercury in biological reference materials by hydride generation, cryofocusing, atomic absorption spectrometry after open focused microwave-assisted alkaline digestion. J. Anal. At. Spectrom. 1997, 12, 743–750. [Google Scholar] [CrossRef]
- Cizdziel, J.V.; Hinners, T.A.; Heithmar, E.M. Determination of total mercury in fish tissues using combustion atomic absorption spectrometry with gold amalgamation. Water Air Soil Pollut. 2002, 135, 355–370. [Google Scholar] [CrossRef]
- Van Griethuysen, C.; Gillissen, F.; Koelmans, A.A. Measuring acid volatile sulphide in floodplain lake sediments: Effect of reaction time, sample size and aeration. Chemosphere 2002, 47, 395–400. [Google Scholar] [CrossRef]
- Maggi, C.; Berducci, M.T.; Bianchi, J.; Giani, M.; Campanella, L. Methylmercury determination in marine sediment and organisms by Direct Mercury Analyser. Anal. Chim. Acta 2009, 614, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; No. 9; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- The Ministry of Environmental Protection of the People’s Republic of China. Chinese Environmental Quality Standards for Surface Water (GB3838-2002); The Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2002. [Google Scholar]
- Zhang, S.Q.; Wang, Q.C.; Zhao, X.M.; Zhang, X.W.; Zheng, D.M. Characteristics of mercury pollution in rivers of different pollution sources in Huludao. Geol. Geochem. 2008, 36, 225–230. [Google Scholar]
- Canário, J.; Branco, V.; Vale, C. Seasonal variation of monomethylmercury concentrations in surface sediments of the Tagus Estuary (Portugal). Environ. Pollut. 2007, 148, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yan, W.; Wang, W.; Chen, Z. Pollution of heavy metals in the Pearl Estuary and its assessment of potential ecological risk. Mar. Environ. Sci. 2002, 21, 34–38. (In Chinese) [Google Scholar]
- Li, X. Distribution and environmental quality assessment of heavy metals in sediments of Daya Bay, Guangdong. Geoscience 2003, 30, 429–437. (In Chinese) [Google Scholar]
- Zhang, C.; Chen, Z.; Bi, C.; Shi, G. The distribution of Hg and as in water and sediment of the drinking water source area of the Huangpu River. Acta Sci. Circumstantiae 2007, 28, 1455–1462. (In Chinese) [Google Scholar]
- Wu, H. Distribution of Methylmercury and Its Microbial Methylation in Major Mangrove Wetlands in China. Master’s Thesis, Xiamen University, Xiamen, China, 2009. (In Chinese). [Google Scholar]
- Zhu, H.; Yan, B.; Zhang, F.; Lu, Y.; Wang, L. Contribution of main components of sediments to mercury adsorption in Songhua River. Environ. Chem. 2010, 29, 865–869. (In Chinese) [Google Scholar]
- Baeyens, W.; Leermakers, M. Elemental mercury concentrations and formation rates in the Scheldt estuary and the North Sea. Mar. Chem. 1998, 60, 257–266. [Google Scholar] [CrossRef]
- Kwokal, Z.; Bilinski, S.F.; Bilinski, H. A comparison of anthropogenic mercury pollution in Kastela Bay (Croatia) with pristine estuaries in Ore (Sweden) and Krka (Croatia). Pergamon Mar. Pollut. Bull. 2002, 44, 1152–1169. [Google Scholar] [CrossRef]
- Ding, X.; Chen, L.; Zhang, W.; Xu, Z.; Peng, X.; Shang, L. Preliminary study on pollution status and assessment of mercury in sediment from the Beijiang River. J. Agro Environ. Sci. 2010, 29, 357–362. [Google Scholar]
- Burton, G.A.; Green, A.; Baudo, R.; Forbes, V.; Nguyen, L.T.; Janssen, C.R.; Kukkonen, J.; Leppanen, M.; Maltby, L.; Soares, A.; et al. Characterizing sediment acid volatile sulphide concentrations in European streams. Environ. Toxicol. Chem. 2007, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.E.; Fu, G.; Deng, B. Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ. Toxicol. Chem. 1993, 12, 1441–1453. [Google Scholar] [CrossRef]
- Fang, T.; Li, X.; Zhang, G. Acid volatile sulfide and simultaneously extracted metals in the sediment cores of the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2005, 61, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, X.; Warren, A.; Zhang, L.; Xu, H. Functional diversity of benthic ciliate communities in response to environmental gradients in a wetland of Yangtze Estuary, China. Mar. Pollut. Bull. 2018, 127, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Orihel, D.M.; Paterson, M.J.; Blanchfield, P.J.; Bodaly, R.A.; Hintelmann, H. Experimental evidence of a linear relationship between inorganic mercury loading and methylmercury accumulation by aquatic biota. Environ. Sci. Technol. 2007, 41, 4952–4958. [Google Scholar] [CrossRef]
- Wang, W.X.; Stupakoff, I.; Gagnon, C.; Fisher, N.S. Bioavailability of inorganic and methylmercury to a marine deposit-feeding polychaete. Environ. Sci. Technol. 1998, 32, 2564–2571. [Google Scholar] [CrossRef]
- Harding, G.; Dalziel, J.; Vass, P. Bioaccumulation of methylmercury within the marine food web of the outer Bay of Fundy, Gulf of Maine. PLoS ONE 2018, 13, e0197220. [Google Scholar] [CrossRef] [PubMed]
- Chételat, J.; Richardson, M.C.; MacMillan, G.A.; Amyot, M.; Poulain, A.J. Ratio of Methylmercury to Dissolved Organic Carbon in Water Explains Methylmercury Bioaccumulation Across a Latitudinal Gradient from North-Temperate to Arctic Lakes. Environ. Sci. Technol. 2017, 52, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.M.; Johs, A.; Podar, M.; Bridou, R.; Hurt, R.A.; Smith, S.D.; Tomanicek, S.J.; Qian, Y.; Brown, S.D.; Brandt, C.C.; et al. The genetic basis for bacterial mercury methylation. Science 2013, 339, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Fardeau, M.L.; Fauque, G.D. Hydrogen sulfide: A toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. In The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment; Springer: Dordrecht, The Netherlands, 2014; pp. 237–277. [Google Scholar]
- Fritz, G.; Schiffer, A.; Behrens, A.; Büchert, T.; Ermler, U.; Kroneck, P.M. Living on Sulfate: Three-Dimensional Structure and Spectroscopy of Adenosine 5-Phosphosulfate Reductase and Dissimilatory Sulfite Reductase. In Microbial Sulfur Metabolism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–23. [Google Scholar]
- Liu, J.; Valsaraj, K.T.; Delaune, R.D. Inhibition of mercury methylation by iron sulfides in an anoxic sediment. Environ. Eng. Sci. 2009, 26, 833–840. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P.; Heyes, A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol. 1999, 33, 951–957. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhou, Q.; Huang, J.; Vymazal, J.; Kuschk, P. Sulfate removal and sulfur transformation in constructed wetlands: The roles of filling material and plant biomass. Water Res. 2016, 102, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Rees, G.N.; Baldwin, D.S.; Watson, G.O.; Hall, K.C. Sulfide formation in freshwater sediments, by sulfate-reducing microorganisms with diverse tolerance to salt. Sci. Total Environ. 2010, 409, 134–139. [Google Scholar] [CrossRef]
- Compeau, G.C.; Bartha, R. Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 1985, 50, 498–502. [Google Scholar]



| Site | Water Depth (cm) | Transparency (cm) | Temperature (°C) | Dissolved Oxygen (mg/L) | pH | CODCr (mg/L) |
|---|---|---|---|---|---|---|
| A | 14 | 14 | 22.9 | 5.3 | 7.2 | 20.3 |
| B | 20 | 15 | 22.7 | 4.3 | 7.0 | 32.3 |
| C | 25 | 0 | 23.1 | 2.9 | 7.2 | 25.6 |
| D | 50 | 0 | 22.7 | 3.2 | 7.0 | 21.7 |
| E | 30 | 20 | 22.7 | 2.3 | 6.7 | 39.7 |
| F | 88 | 10 | 21.7 | 0 | 5.8 | 80.3 |
| G | 80 | 10 | 22.1 | 3.6 | 5.9 | 76.9 |
| H | 15 | 15 | 22.2 | 2.9 | 7.1 | 49.1 |
| I | 90 | 15 | 22.3 | 4.7 | 6.9 | 29.2 |
| J | 12 | 0 | 23.1 | 4.3 | 6.6 | 29.1 |
| K | 46 | 17 | 22.1 | 2.7 | 6.8 | 51.2 |
| L | 23 | 23 | 22.2 | 3.7 | 6.9 | 22.6 |
| M | 100 | 20 | 21.9 | 2.5 | 7.1 | 31.2 |
| Site | HgTot (μg/g) | HgSEM (μg/g) | MeHg (ng/g) | AVS (μg/g) | SRB (MPN/g (d.w.)) | OC (%) | Eh (mV) |
|---|---|---|---|---|---|---|---|
| A | 3.631 ± 1.051 | 0.037 ± 0.025 | 0.672 ± 0.136 | 402.56 ± 99.84 | 4.46 × 104 | 4.44 ± 1.72 | −144 ± 10 |
| B | 2.247 ± 0.134 | 0.091 ± 0.023 | 0.598 ± 0.108 | 536.96 ± 263.04 | 2.07 × 103 | 8.53 ± 2.83 | −106 ± 5 |
| C | 1.976 ± 0.976 | 0.052 ± 0.025 | 0.566 ± 0.037 | 294.08 ± 144.32 | 4.11 × 103 | 7.28 ± 0.16 | −146 ± 2 |
| D | 4.450 ± 1.580 | 0.095 ± 0.075 | 0.286 ± 0.053 | 404.80 ± 97.6 | 1.67 × 103 | 7.95 ± 0.88 | −130 ± 21 |
| E | 1.073 ± 0.308 | 0.545 ± 0.461 | 0.150 ± 0.019 | 588.80 ± 344.96 | 1.87 × 103 | 4.85 ± 0.84 | −126 ± 15 |
| F | 1.744 ± 0.716 | 0.090 ± 0.046 | 0.213 ± 0.041 | 278.72 ± 66.88 | 2.08 × 103 | 4.33 ± 0.15 | −108 ± 6 |
| G | 2.379 ± 0.234 | 0.099 ± 0.031 | 0.260 ± 0.070 | 484.8 ± 78.4 | 4.40 × 104 | 6.83 ± 0.58 | −121 ± 20 |
| H | 4.244 ± 0.787 | 0.045 ± 0.014 | 0.347 ± 0.056 | 351.36 ± 32.64 | 3.01 × 103 | 11.14 ± 4.40 | −154 ± 4 |
| I | 2.766 ± 1.237 | 0.624 ± 0.371 | 0.591 ± 0.187 | 408 ± 40 | 2.07 × 103 | 3.21 ± 1.06 | −134 ± 17 |
| J | 3.068 ± 0.593 | 0.025 ± 0.010 | 0.622 ± 0.051 | 550.08 ± 224 | 4.56 × 104 | 7.18 ± 0.83 | −118 ± 16 |
| K | 2.830 ± 1.018 | 0.075 ± 0.034 | 0.081 ± 0.005 | 140.48 ± 45.12 | 2.37 × 103 | 5.05 ± 0.73 | −150 ± 17 |
| L | 2.182 ± 0.687 | 0.109 ± 0.019 | 0.115 ± 0.032 | 253.76 ± 67.20 | 3.19 × 103 | 4.91 ± 0.66 | −152 ± 9 |
| M | 1.122 ± 0.261 | 0.097 ± 0.054 | 1.554 ± 0.026 | 652.16 ± 281.6 | 5.20 × 104 | 7.51 ± 1.71 | −155 ± 8 |
| Study Area | Sedimentary Hg (µg/g) | Reference | |
|---|---|---|---|
| Range | Average | ||
| Cishan River (China) | 0.344–132.500 | 52.450 | [40] |
| Cishan River (China) | 0.037–16.070 | 9.241 | [40] |
| Cishan River (China) | 0.215–19.550 | 5.126 | [40] |
| Tagus Estuary (Portugal) | 0.01–66.7 | [41] | |
| Pearl River Estuary (China) | 0.20–0.72 | 0.354 | [42] |
| Daya Bay (China) | 0.03–0.12 | 0.07 | [43] |
| Huangpu River (China) | 0.0479–0.4169 | 0.1488 | [44] |
| Fugong Mangrove area (China) | 0.17–0.21 | [45] | |
| Shenzhen Mangrove area (China) | 0.17–0.19 | [45] | |
| Dongzhaigang Mangrove area (China) | 0.02–0.65 | [45] | |
| Sanya Mangrove area (China) | 0.02–0.31 | [45] | |
| Daguansha Mangrove area (China) | 0.01–0.04 | [45] | |
| Songhua River (China) | 0.013–1.543 | 0.610 | [46] |
| Scheldt Estuary (Belgium) | 0.14–0.18 | [47] | |
| Öre Estuary (Sweden) | 0.03–0.12 | [48] | |
| North River (China) | 0.074–3.517 | 0.61 | [49] |
| Southwest Creek (China) | 1.073–4.450 | 2.593 | This study |
| Phylum | Species | Population Density of Benthos at Each Site (ind/m2) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | D | F | J | K | L | M | ||
| Mollusc | Hippeutis cantori | 122 | 0 | 0 | 0 | 30 | 53 | 0 |
| Cipangopaludina chinesis | 0 | 0 | 0 | 10 | 10 | 13 | 0 | |
| Bellamya aeruginosa | 41 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Alocinma longicornis | 10 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Katayama nosophora Robson | 41 | 0 | 0 | 10 | 0 | 0 | 10 | |
| Radix swinhoei | 0 | 0 | 0 | 0 | 10 | 0 | 0 | |
| Corbicula fluminea | 10 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Annelid | Branchiura sowerbyi | 0 | 467 | 193 | 152 | 20 | 0 | 203 |
| Limnodrilus hoffmeisteri | 913 | 14,138 | 7505 | 3550 | 2901 | 13 | 7677 | |
| Nais communis | 0 | 0 | 0 | 0 | 0 | 13 | 0 | |
| Tylorrhynchus heterochaetus | 0 | 20 | 0 | 0 | 0 | 0 | 0 | |
| Arthropod | Tanypus chinensis | 0 | 0 | 0 | 0 | 0 | 13 | 0 |
| Ephacerella sp. | 0 | 0 | 0 | 0 | 0 | 13 | 0 | |
| Platyhelminthe | Planocera reticulate | 0 | 0 | 0 | 0 | 0 | 13 | 0 |
| Dugesia gonocephala | 0 | 0 | 0 | 0 | 0 | 26 | 0 | |
| Total number of organisms | 1137 | 14,625 | 7698 | 3722 | 2971 | 157 | 7890 | |
| Total number of species | 6 | 3 | 2 | 4 | 5 | 8 | 3 | |
| Shannon–Wiener biodiversity index | 1.07 | 0.22 | 0.17 | 0.31 | 0.21 | 2.74 | 0.17 | |
| Parameter | AVS | SRB | OC | HgTot | HgSEM | Eh | MeHg |
|---|---|---|---|---|---|---|---|
| AVS | 1.00 | ||||||
| SRB | 0.54 | 1.00 | |||||
| OC | 0.23 | 0.05 | 1.00 | ||||
| HgTot | −0.29 | −0.07 | 0.34 | 1.00 | |||
| HgSEM | 0.27 | −0.27 | −0.51 | −0.31 | 1.00 | ||
| Eh | 0.31 | −0.06 | −0.09 | −0.15 | 0.14 | 1.00 | |
| MeHg | 0.60 * | 0.62 * | 0.12 | −0.23 | −0.08 | −0.22 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, F.; Huang, W.; Li, Z.; Chen, M. Evaluation of Mercury Transformation and Benthic Organisms Uptake in a Creek Sediment of Pearl River Estuary, China. Water 2019, 11, 1308. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061308
Chen L, Li F, Huang W, Li Z, Chen M. Evaluation of Mercury Transformation and Benthic Organisms Uptake in a Creek Sediment of Pearl River Estuary, China. Water. 2019; 11(6):1308. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061308
Chicago/Turabian StyleChen, Long, Feng Li, Wenrou Huang, Zhi Li, and Mingguang Chen. 2019. "Evaluation of Mercury Transformation and Benthic Organisms Uptake in a Creek Sediment of Pearl River Estuary, China" Water 11, no. 6: 1308. https://0-doi-org.brum.beds.ac.uk/10.3390/w11061308