Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ACF/TiO2 Composite Adsorbents
2.3. Characterization
2.4. Adsorption and Photocatalytic Degradation Studies
3. Results and Discussion
3.1. Characterization of ACF/TiO2 Composite Adsorbents
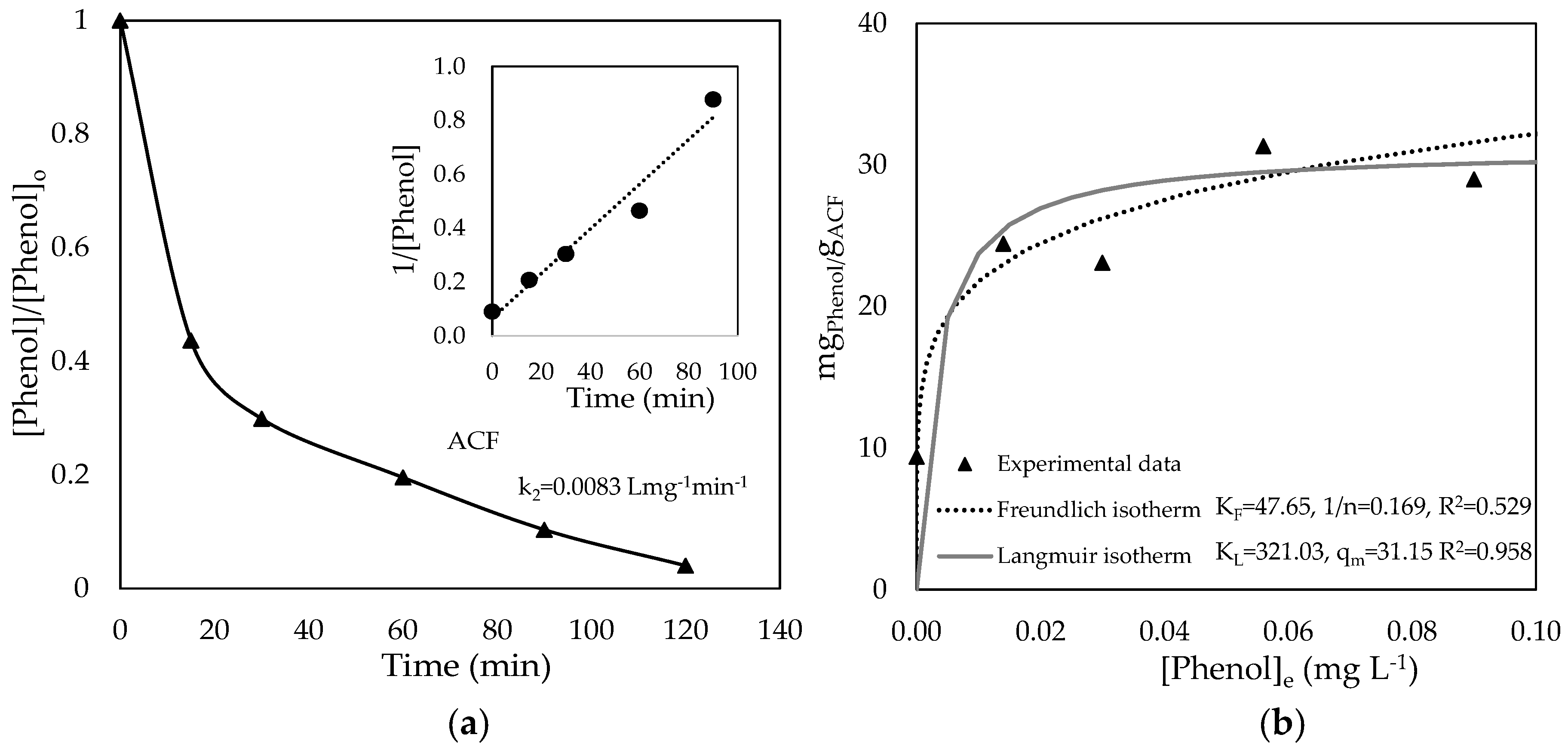
3.2. Phenol Adsorption on Original ACF and ACF/TiO2 Composites
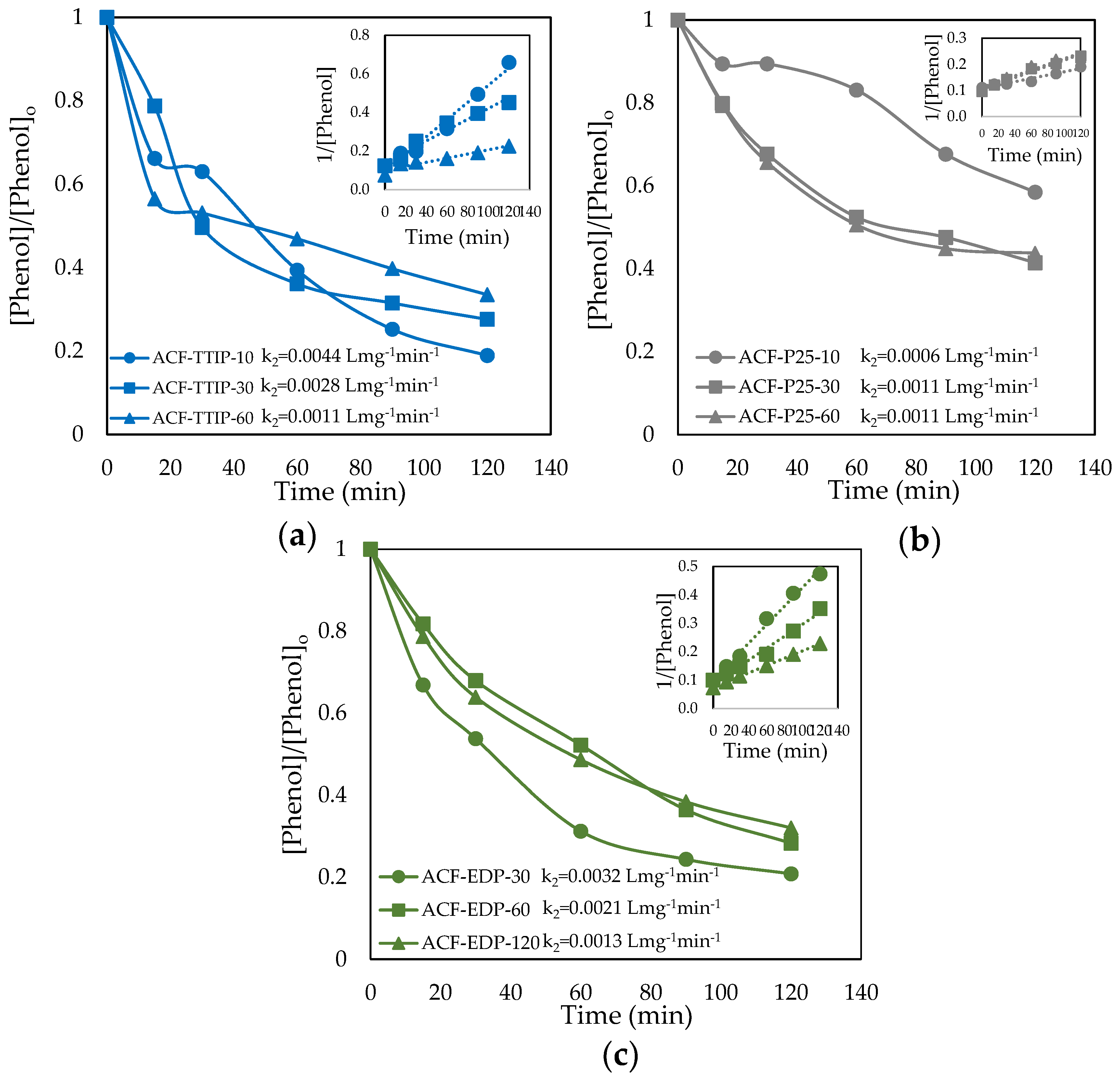
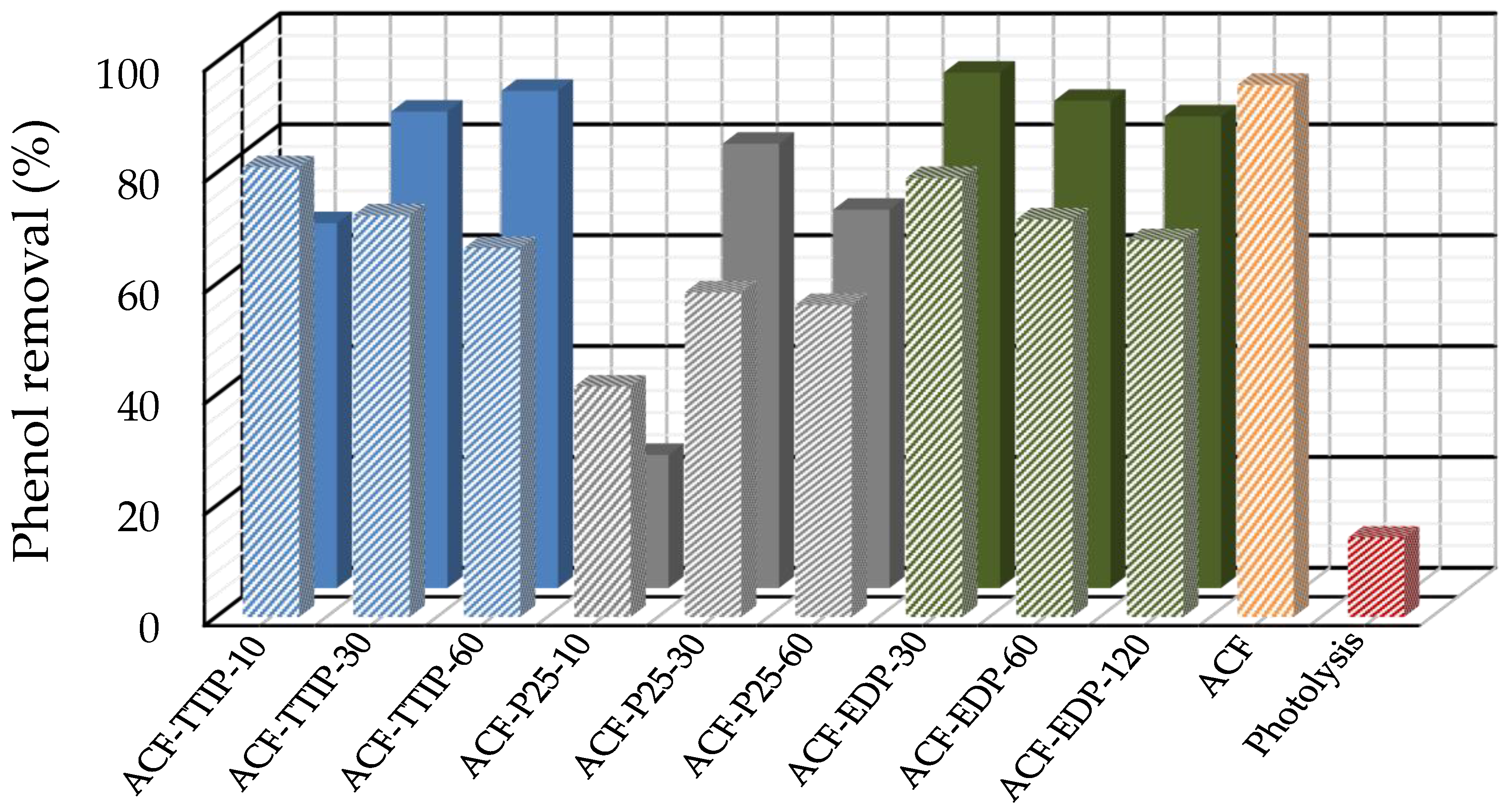
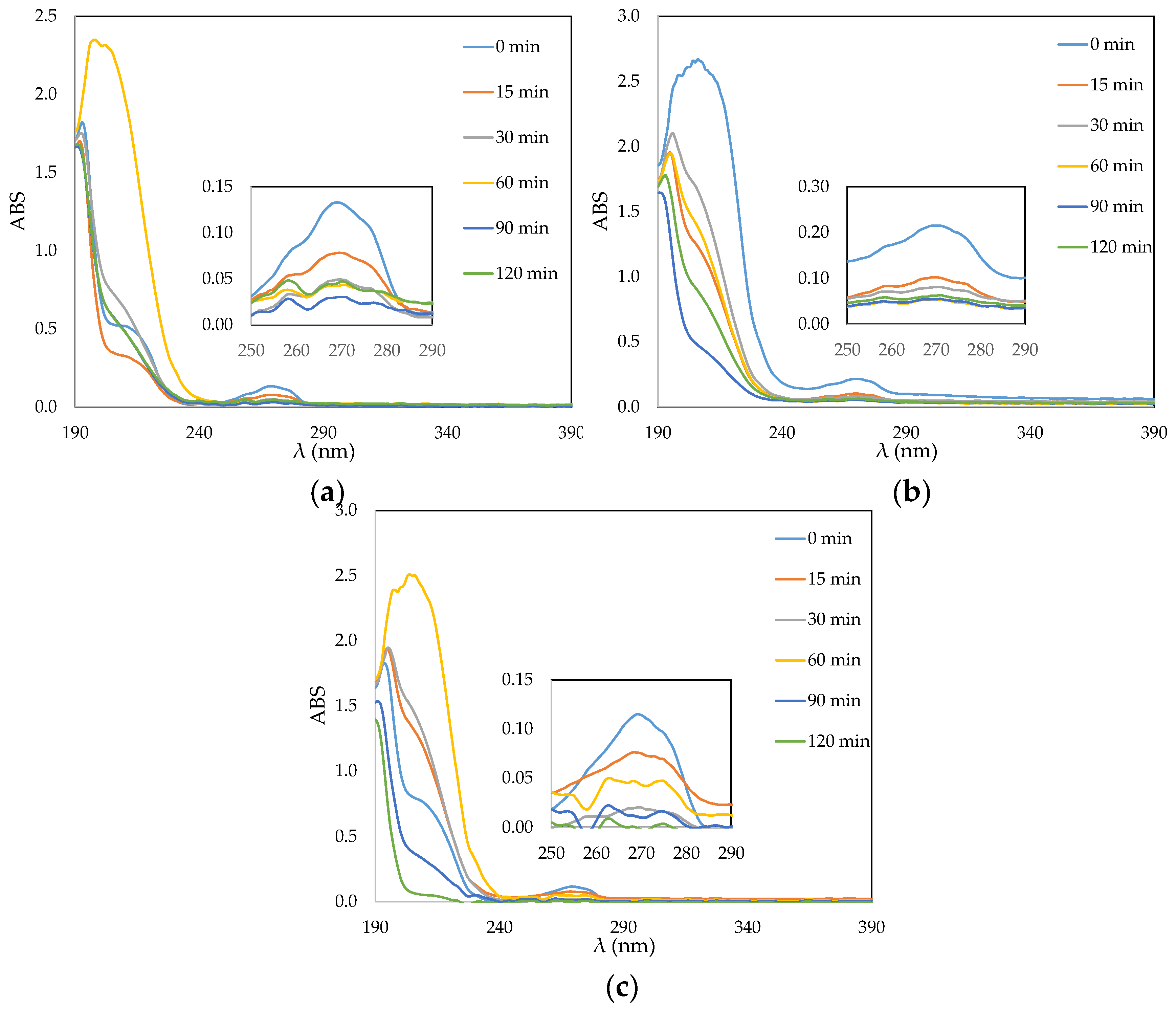
3.3. Phenol Photocatalytic Degradation by ACF/TiO2 Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Voumard, M.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Micropollutant degradation, bacterial inactivation and regrowth risk in wastewater effluents: Influence of the secondary (pre)treatment on the efficiency of Advanced Oxidation Processes. Water Res. 2016, 102, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Plakas, K.V.; Sarasidis, V.C. How far are we from large scale PMR applications. In Current Trends and Future Developments on (Bio-) Membranes: Renewable Energy Integrated with Membrane Operations, 1st ed.; Mozia, S., Molinari, R., Basile, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 233–295. [Google Scholar]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Activated carbon fibers from NOMEX by chemical activation with phosphoric acid. Carbon 2004, 42, 1419–1426. [Google Scholar]
- Rabbi, A.; Dadashian, F. Simultaneous improvement in tensile strength and adsorption capacity of activated carbon fibers during stabilization and activation of acrylic fibers. Diam. Relat. Mater. 2019, 95, 174–184. [Google Scholar] [CrossRef]
- Hou, Y.; Qu, J.; Zhao, X.; Lei, P.; Wan, D.; Huang, C.P. Electro-photocatalytic degradation of acid orange II using a novel TiO2/ACF photoanode. Sci. Total. Environ. 2009, 407, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, B.-K. Growth of TiO2 nano-wall on activated carbon fibers for enhancing the photocatalytic oxidation of benzene in aqueous phase. Catal. Today 2017, 287, 113–121. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Enhancement effect of TiO2 immobilized on activated carbon filter for the photodegradation of pollutants at typical indoor air level. Appl. Catal. B Environ. 2003, 44, 191–205. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Decontamination of textile wastewater via TiO2/activated carbon composite materials. Adv. Colloid Interface Sci. 2010, 159, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, X.; Li, W.; Gao, J.; Xue, H.; Li, R.K.Y.; Mai, Y.-W. TiO2 nanoparticle decorated carbon nanofibers for removal of organic dyes. Colloid Surf. A 2018, 549, 205–211. [Google Scholar] [CrossRef]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Riegel, G.; Bolton, J.R. Photocatalytic efficiency variability in TiO2 particles. J. Phys. Chem. 1995, 99, 4215–4224. [Google Scholar] [CrossRef]
- Stafford, U.; Gray, K.A.; Kamat, P.V.; Varma, A. An in situ diffuse reflectance FTIR investigation of photocatalytic degradation of 4-chlorophenol on a TiO2 powder surface. Chem. Phys. Lett. 1993, 205, 55–61. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; McKinney, B.; Rouse, M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Pereira, L.A.; Couto, A.B.; de Lima Almeida, D.A.; Ferreira, N.G. The influence of TiO2 amount on the photoactivity response for the novel TiO2/BDD/carbon fiber ternary composite. Diam. Relat. Mater. 2017, 75, 18–24. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, J.; Wu, P.; Ji, X. Immobilization of TiO2 films on activated carbon fiber and their photocatalytic degradation properties for dye compounds with different molecular size. Catal. Commun. 2008, 9, 1846–1850. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Shi, Z. Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants. Particuology 2010, 8, 272–278. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Y.; Ni, Q.-Q. Preparation and performance of photocatalytic TiO2 immobilized on palladium-doped carbon fibers. Appl. Surf. Sci. 2011, 257, 2275–2280. [Google Scholar] [CrossRef]
- Wang, C.; Yue, R.; Wang, H.; Zou, C.; Du, J.; Jiang, F.; Du, Y.; Yang, P.; Wang, C. Dendritic Ag@Pt core-shell catalyst modified with reduced graphene oxide and titanium dioxide: Fabrication, characterization, and its photo-electrocatalytic performance. Int. J. Hydrog. Energy 2014, 39, 5764–5771. [Google Scholar] [CrossRef]
- Wang, Z.; Yoshinaga, K.; Bu, X.R.; Zhang, M. Low temperature fabrication & photocatalytical activity of carbon fiber-supported TiO2 with different phase compositions. J. Hazard. Mater. 2015, 290, 134–141. [Google Scholar]
- Hu, H.; Pang, B.; Zhu, Y.; Fu, Y. Preparation of titanium dioxide immobilized on carbon fibers annealed in steam ambient and their photocatalytic properties. Text. Res. J. 2017, 87, 2233–2241. [Google Scholar] [CrossRef]
- Yu, H.; Quan, X.; Chen, S.; Zhao, H. TiO2-multiwalled carbon nanotube heterojunction arrays and their charge separation capability. J. Phys. Chem. C 2007, 111, 12987–12991. [Google Scholar] [CrossRef]
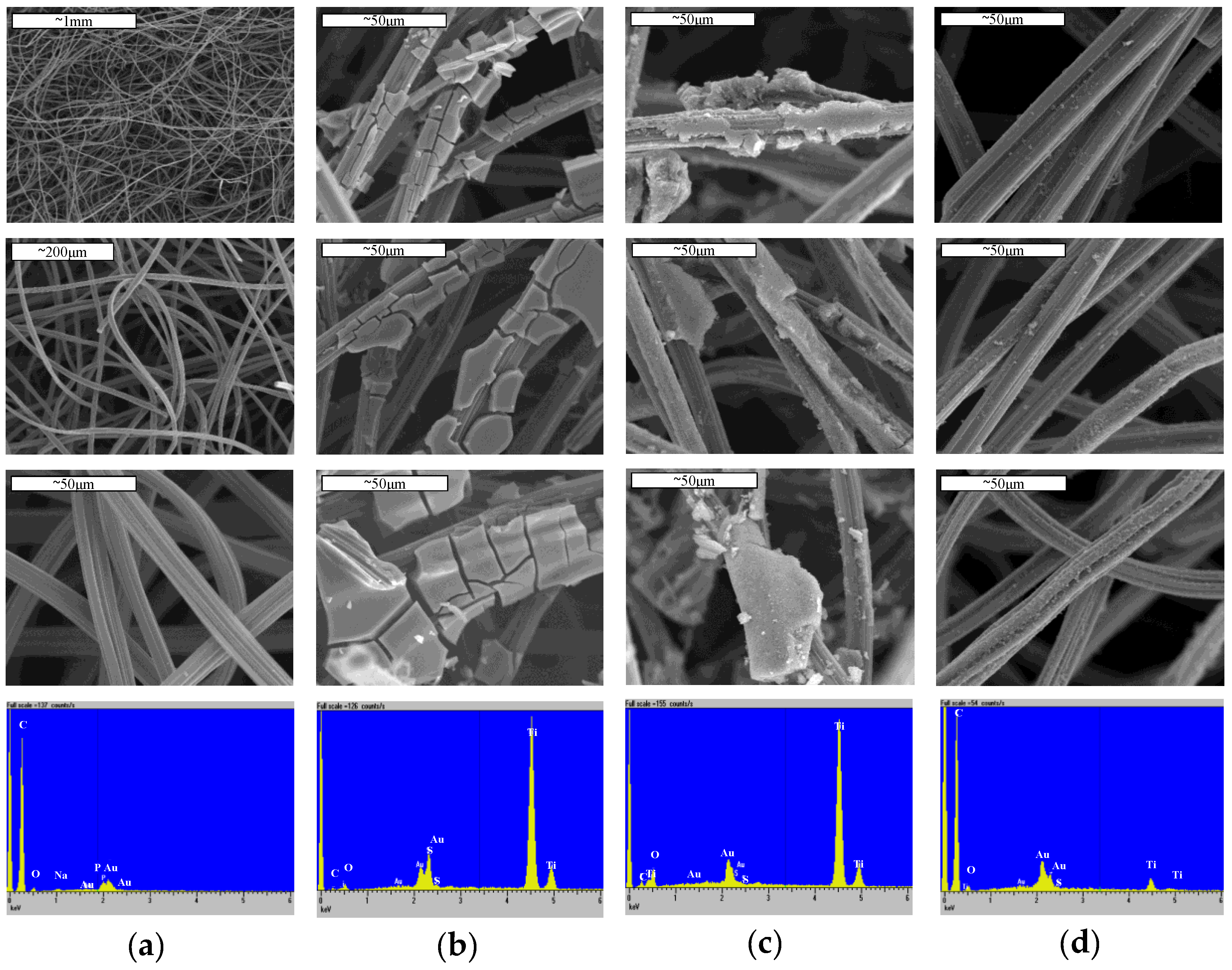
| Method | Substrate | Results | Ref. |
|---|---|---|---|
| Sol-gel adsorption | Commercial activated carbon fiber | Calcination temperature greatly affected the structure morphologies of TiO2 films; rapid removal of Methyl Orange and acid fuchsine | [25] |
| Dip-coating | Viscose rayon-based carbon fibers (activated carbon fiber) | Photocatalytic degradation of Methyl Orange and phenol described by first-order kinetics; effective reuse of the activated carbon fiber (ACF)-supported photocatalyst | [26] |
| Dip-coating | Polyacrylonitrile carbon fiber (modified with Pd) | TiO2/Pd-carbon fiber (CF) exhibited 70% higher catalytic efficiency for Acid Orange II removal than TiO2/CF | [27] |
| In-situ deposition | Commercial carbon fiber | Fabricated three-dimensional electrode composed of dendritic Ag@Pt core-shell catalyst, reduced graphene oxide, TiO2 spheres, and carbon fiber exhibited improved photo-electrocatalytic performance for methanol oxidation compared to other comparative electrodes | [28] |
| Mixing with Ti(OH)4 and H2O2 | Commercial carbon fiber | The photocatalytic degradation of Methyl Orange found to be phase composition-dependent and pH dependent | [29] |
| Dip-coating and annealing under superheated steam | Polyacrylonitrile carbon fiber | TiO2/CF composites achieved up to 98.7% degradation rate of Acid Orange II after 2.5 h of irradiation | [30] |
| Hydrothermal | Commercial activated carbon fiber | The composite ACF/TiO2 presented good uniformity, high crystallinity, and large benzene photo-oxidation and sorption affinity | [14] |
| Ultra-sonication induced adsorption or electrospinning | Polyacrylonitrile nanofiber | TiO2 nanoparticles decorated carbon nanofibers, prepared by ultra-sonication, presented higher Methylene Blue adsorption capacity and photo-catalytic efficiency than those obtained by electrospinning | [18] |
| ACF Pretreatment | Electrolysis of ACF specimens at a fixed potential of 2.0 V/Ag/AgCl for 30 min in a 0.5 mol L−1 H2SO4 solution followed by overnight drying at 105 °C |
| TiO2 sol Preparation | 1. ΤΤΙP in Milli-Q water (a) Addition of 36 mL titanium (IV) isopropoxide (TTIP) and 3.8 mL HNO3 solution in 400 mL Milli-Q water, (b) gentle stirring for 24 h, (c) heating of the homogeneous solution at 55 °C for 6 h 2. Degussa P-25 in Milli-Q water (a) Addition of 9.71 gr ΤiO2 in 400 mL Milli-Q water, (b) sonication for 30 min 3. Degussa P-25 in methanol (a) Addition of 50 mg TiO2 in 500 mL methanol, (b) sonication for 15 min |
| TiO2 Immobilization | 1. Dip-coating Dipping of ACF specimens in TTIP solution varying the deposition time: (a) 10 s (ACF-TTIP-10), (b) 30 s (ACF-TTIP-30), (c) 60 s (ACF-TTIP-60)Dipping of ACF specimen in Degussa P-25 suspension varying the deposition time: (a) 10 s (ACF-P25-10), (b) 30 s (ACF-P25-30), (c) 60 s (ACF-P25-60) 2. Electrophoretic coating Electrolysis of methanol Degussa P-25 solution under potentiostatic mode at a fixed potential of 10 V/Ag/AgCl varying the electro-deposition (EDP) time: (a) 30 s (ACF-EDP-30), (b) 60 s (ACF-EDP-60), (c) 120 s (ACF-EDP-120) |
| ACF/TiO2 Post-Treatment | Heating at 200 °C for 5 h and storage in desiccator at room temperature for further use |
| Sample | ΒΕΤ Specific Surface Area (m2 g−1) | Mean Pore Volume (cm3 g−1) | Phenol Adsorbed (mgPhenol gadsorbent−1) |
|---|---|---|---|
| ACF | 1155.29 ± 4.93 | 265.39 | 10.16 |
| ACF-TTIP-10 | 852.99 ± 3.41 | 195.95 | 6.20 |
| ACF-TTIP-30 | 868.70 ± 3.77 | 199.56 | 5.59 |
| ACF-TTIP-60 | 726.67 ± 2.70 | 166.93 | 5.45 |
| ACF-P25-10 | 890.26 ± 3.40 | 204.51 | 3.49 |
| ACF-P25-30 | 856.69 ± 3.83 | 196.80 | 5.78 |
| ACF-P25-60 | 872.72 ± 3.43 | 200.48 | 5.47 |
| ACF-EDP-30 | 1123.54 ± 5.48 | 258.10 | 6.94 |
| ACF-EDP-60 | 1121.16 ± 4.90 | 257.55 | 6.82 |
| ACF-EDP-120 | 1180.70 ± 5.59 | 271.22 | 7.93 |
| Sample | Zero Order | 1st Order | 2nd Order | |||
|---|---|---|---|---|---|---|
| k0 (min−1) | R2 | k1 (min−1) | R2 | k2 (L mg−1 min−1) | R2 | |
| ACF-TTIP-10 | 0.0599 | 0.8950 | 0.0098 | 0.9000 | 0.0017 | 0.8948 |
| ACF-TTIP-30 | 0.1084 | 0.9549 | 0.0304 | 0.9664 | 0.0113 | 0.8319 |
| ACF-TTIP-60 | 0.1175 | 0.9680 | 0.0123 | 0.9702 | 0.0015 | 0.9664 |
| ACF-P25-10 | 0.0080 | 0.9651 | 0.0010 | 0.9730 | 0.0001 | 0.9776 |
| ACF-P25-30 | 0.1537 | 0.7632 | 0.0370 | 0.9124 | 0.0115 | 0.9966 |
| ACF-P25-60 | 0.0594 | 0.6865 | 0.0112 | 0.8149 | 0.0024 | 0.9256 |
| ACF-EDP-30 | 0.0015 | 0.8929 | 0.0293 | 0.7812 | 0.0194 | 0.8493 |
| ACF-EDP-60 | 0.0087 | 0.6985 | 0.0244 | 0.8637 | 0.0113 | 0.9365 |
| ACF-EDP-120 | 0.0127 | 0.5192 | 0.0131 | 0.6476 | 0.0045 | 0.7946 |
| No of cycle | 1 | 2 | 3 |
| Phenol removal (%) | 92.7 | 93.7 | 90.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plakas, K.V.; Taxintari, A.; Karabelas, A.J. Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment. Water 2019, 11, 1794. https://0-doi-org.brum.beds.ac.uk/10.3390/w11091794
Plakas KV, Taxintari A, Karabelas AJ. Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment. Water. 2019; 11(9):1794. https://0-doi-org.brum.beds.ac.uk/10.3390/w11091794
Chicago/Turabian StylePlakas, Konstantinos V., Athina Taxintari, and Anastasios J. Karabelas. 2019. "Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment" Water 11, no. 9: 1794. https://0-doi-org.brum.beds.ac.uk/10.3390/w11091794





