Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heterogeneous Fenton Like Oxidation System
2.3. Produced Water Characterization
2.4. Statistical Modelling and Design of Experiments Using Response Surface Methodology (RSM)
3. Results and Discussion
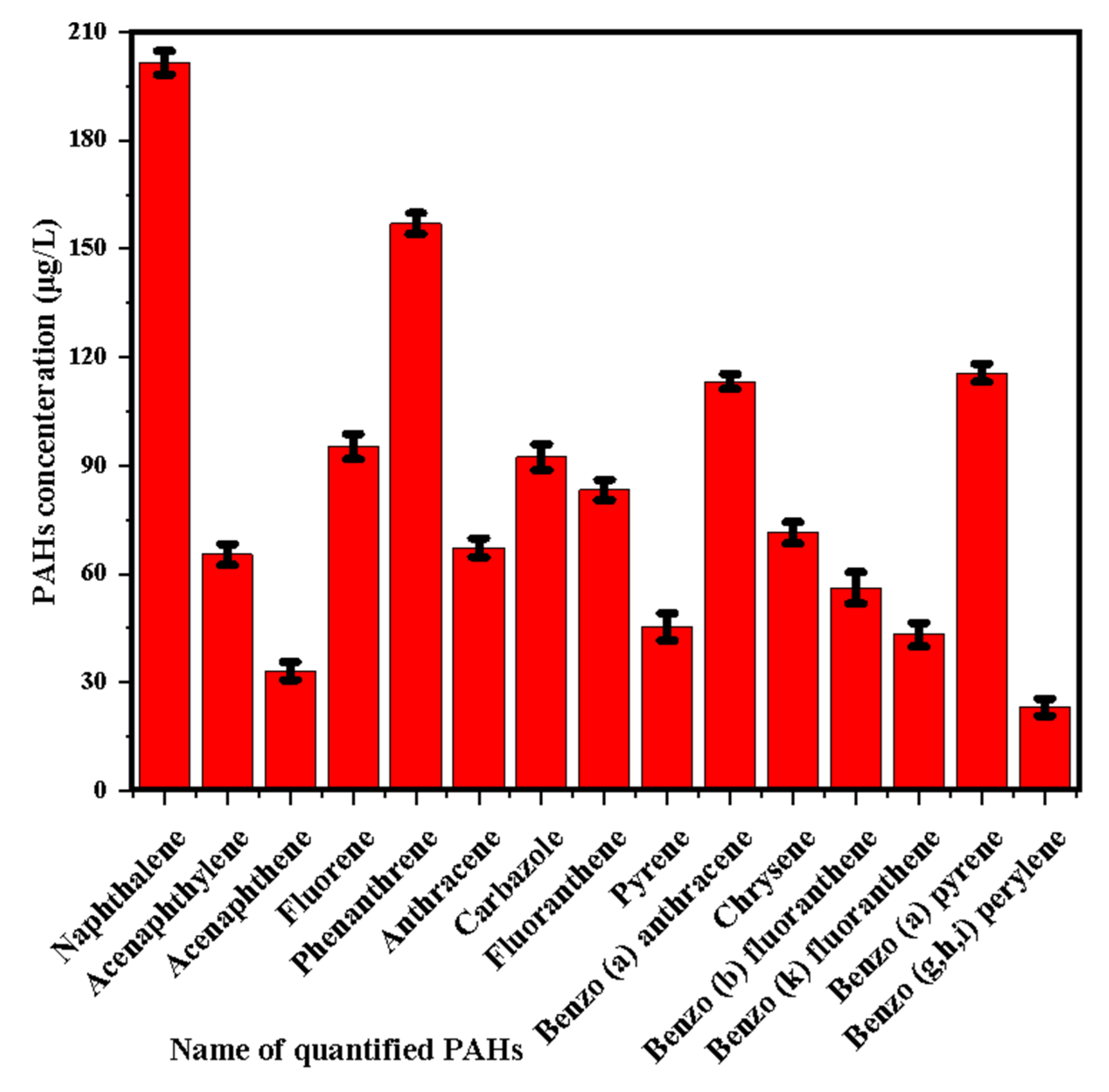
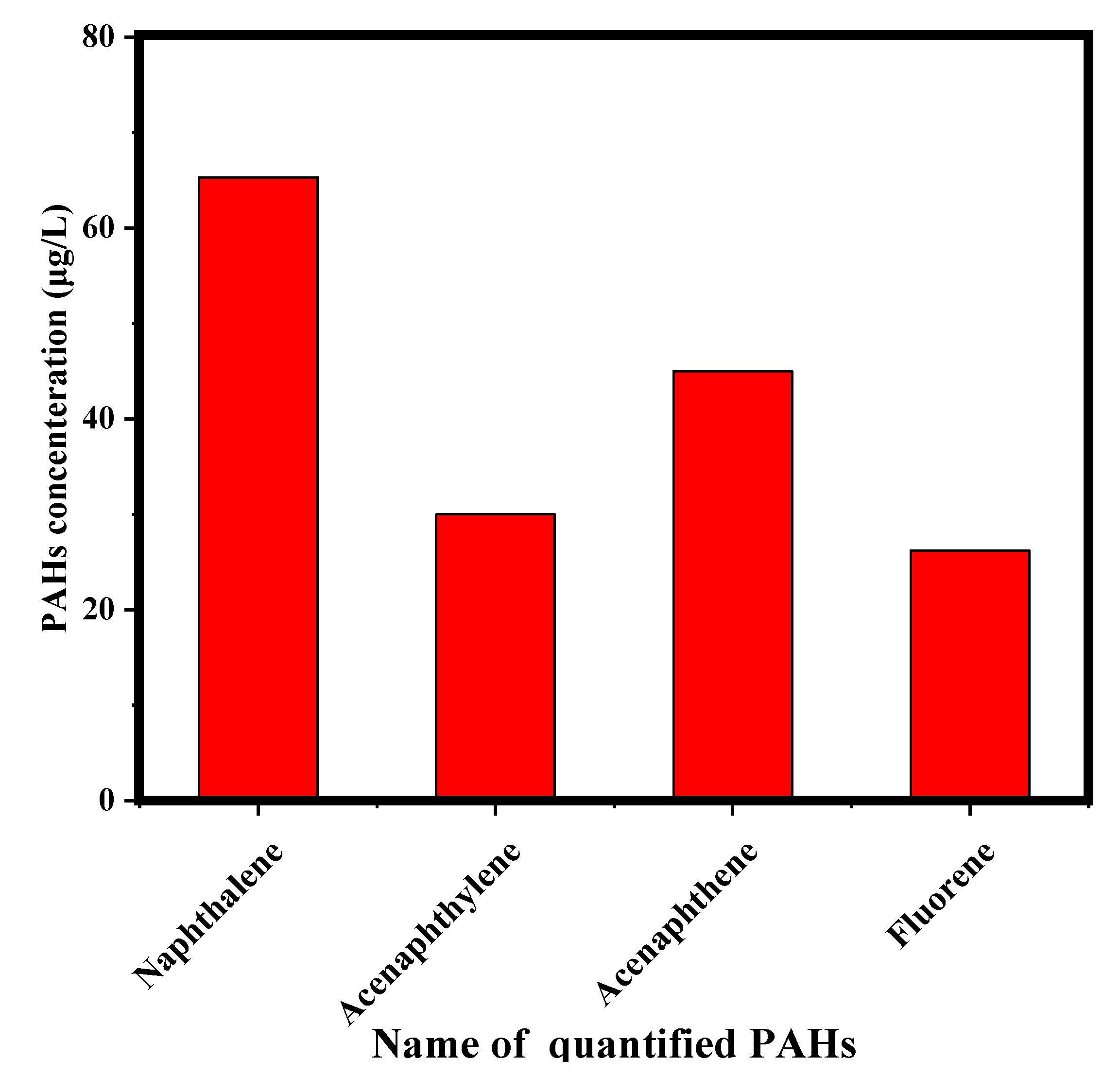
3.1. Produced Water Characteristics
3.2. Preliminary Batch Experiments
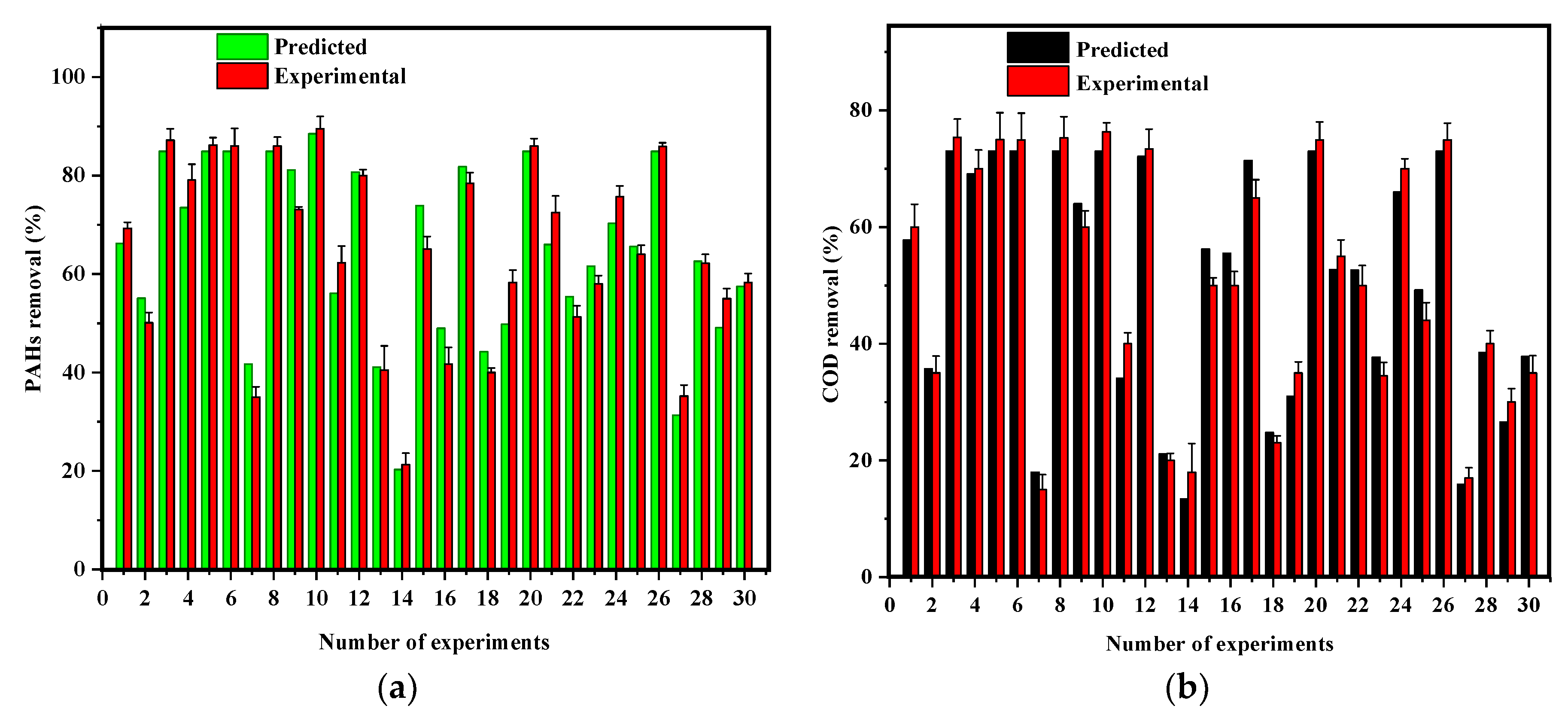
3.3. Batch Experiments Based on RSM Experimental Design
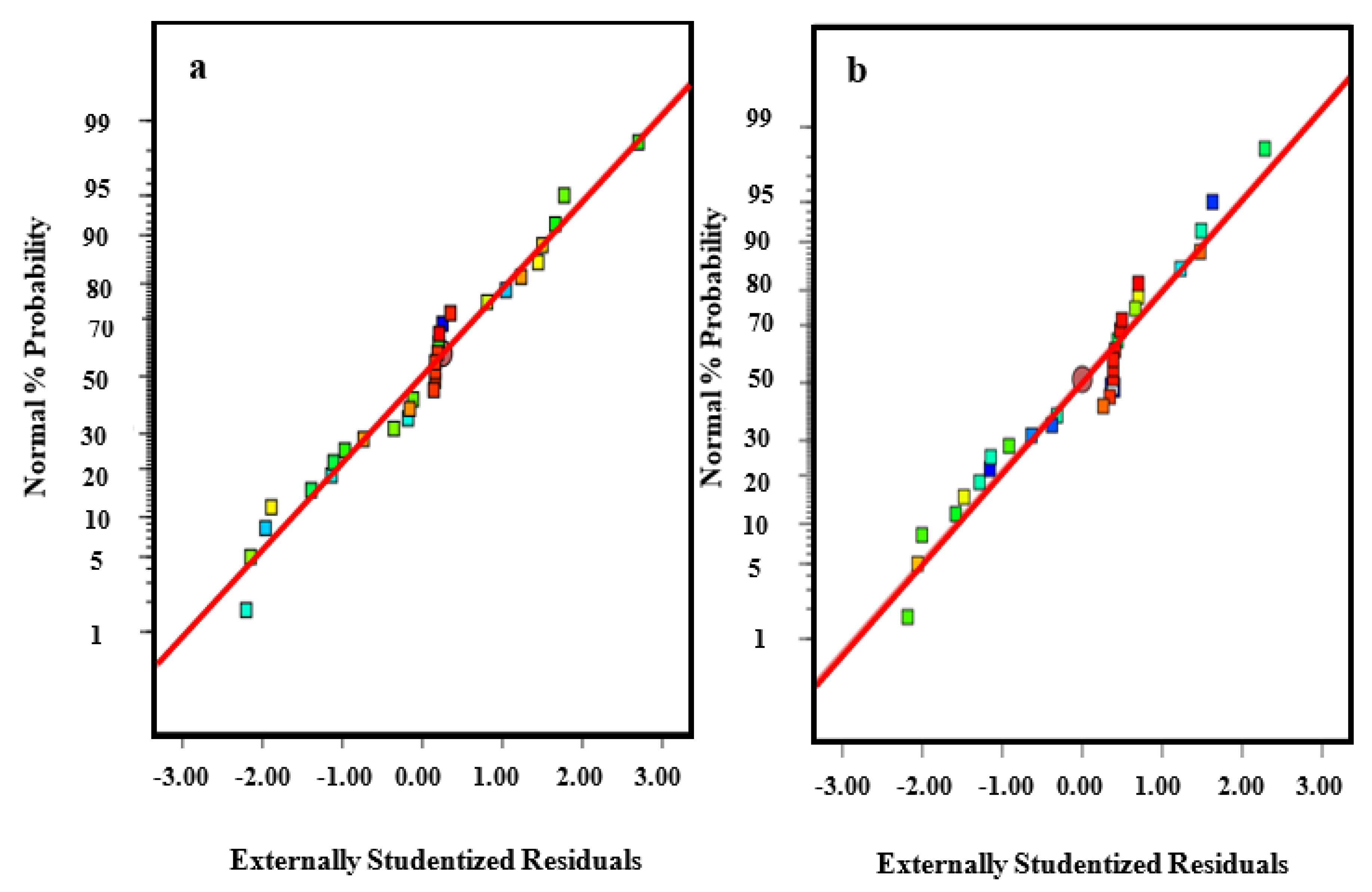
3.4. Analysis of Variance and Fit Summary
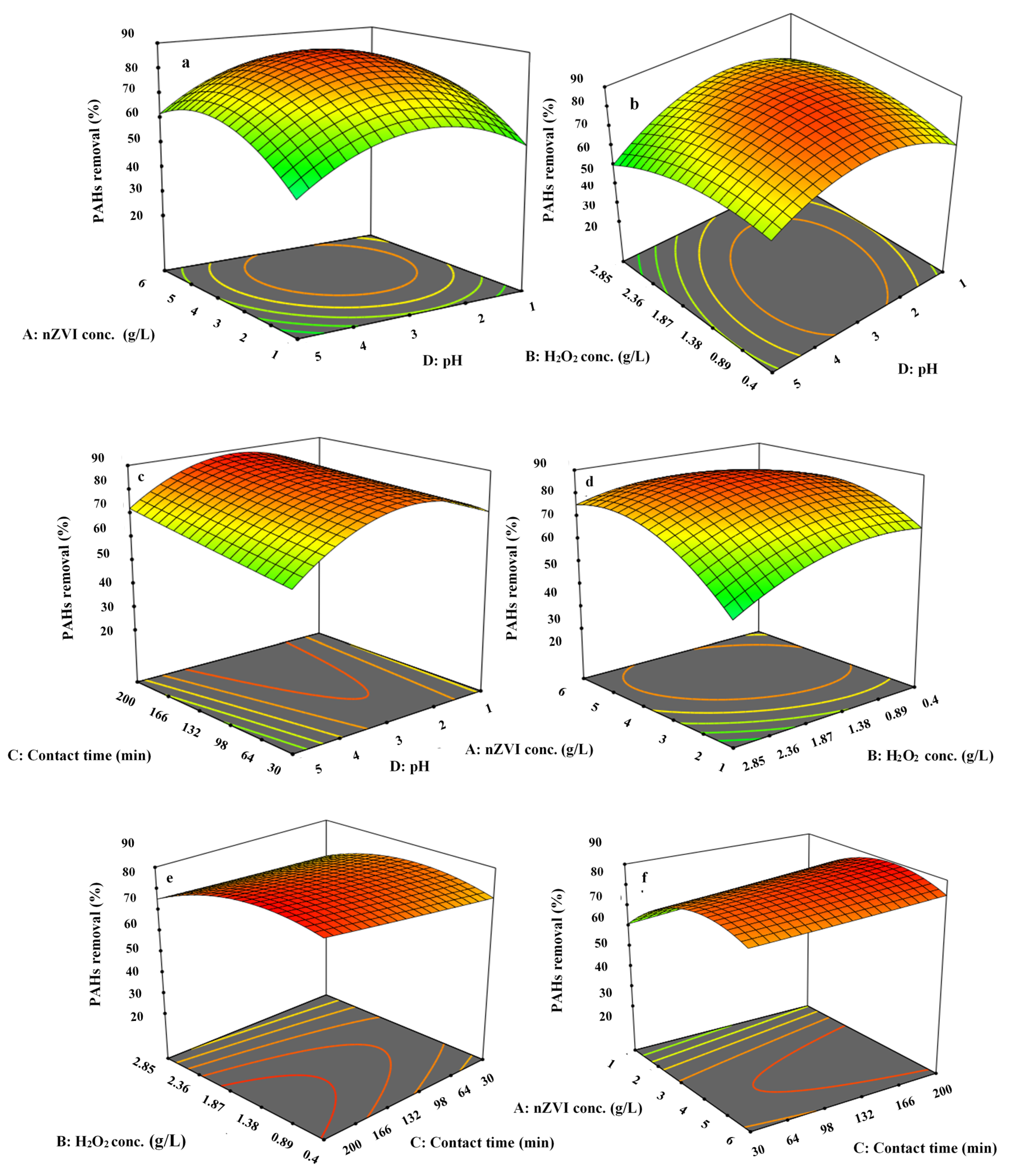
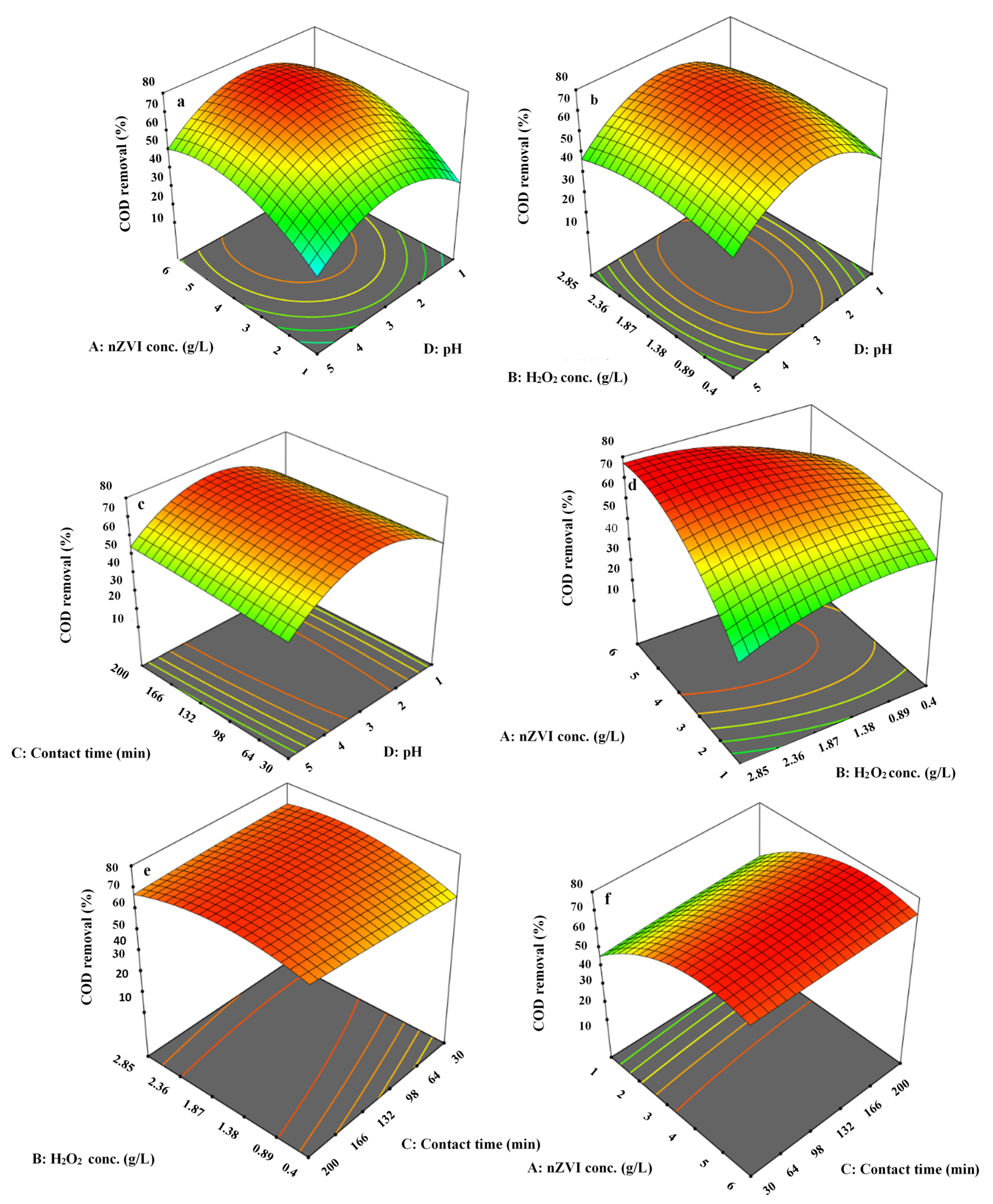
3.5. 3-Dimensional Surface Plots for PAHs and COD Removal Efficiency
3.6. Optimization and Validation of Experimental Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afzal, T.; Isa, M.H.; Ul Mustafa, M.R. Removal of organic pollutants from produced water using Fenton oxidation. In Proceedings of the E3S Web of Conferences, Penang, Malaysia, 18 September 2018; p. 02035. [Google Scholar]
- Haneef, T.; Mustafa, M.R.; Farhan Yasin, H.; Farooq, S.; Hasnain Isa, M.J.E. Study of Ferrate (VI) oxidation for COD removal from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 442, 012007. [Google Scholar] [CrossRef] [Green Version]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Qurban, M.A.; Saleh, T.A. Polycyclic aromatic hydrocarbons extraction and removal from wastewater by carbon nanotubes: A review of the current technologies, challenges and prospects. Process. Saf. Environ. Prot. 2019, 122, 68–82. [Google Scholar] [CrossRef]
- Yaqub, A.; Isa, M.H.; Ajab, H.; Kutty, S.R.; Ezechi, E.H. Polycyclic aromatic hydrocarbons removal from produced water by electrochemical process optimization. Ecol. Chem. Eng. S 2017, 24, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Fakhru, R.A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar]
- Rasool, K.; Shahzad, A.; Lee, D.S. Exploring the potential of anaerobic sulfate reduction process in treating sulfonated diazo dye: Microbial community analysis using bar-coded pyrosequencing. J. Hazard. Mater. 2016, 318, 641–649. [Google Scholar] [CrossRef]
- Saeed, M.O.; Azizli, K.; Isa, M.H.; Bashir, M.J. Application of CCD in RSM to obtain optimize treatment of POME using Fenton oxidation process. J. Water Process. Eng. 2015, 8, 7–16. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: A critical review. J. Clean. Prod. 2019, 237, 1–14. [Google Scholar] [CrossRef]
- Nawaz, M.; Shahzad, A.; Tahir, K.; Kim, J.; Moztahida, M.; Jang, J.; Alam, M.B.; Lee, S.H.; Jung, H.Y.; Lee, D.S. Photo-Fenton reaction for the degradation of sulfamethoxazole using a multi-walled carbon nanotube-NiFe2O4 composite. Chem. Eng. J. 2020, 382, 123053. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Y.; Gao, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem. Eng. J. 2014, 255, 141–148. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J. Hazard. Mater. 2011, 186, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Jarosova, B.; Filip, J.; Hilscherová, K.; Tuček, J.; Šimek, Z.; Giesy, J.P.; Zbořil, R.; Bláha, L. Can zero-valent iron nanoparticles remove waterborne estrogens? J. Environ. Manag. 2015, 150, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [PubMed] [Green Version]
- Zhang, W.; Gao, H.; He, J.; Yang, P.; Wang, D.; Ma, T.; Xia, H.; Xu, X. Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: Optimization of operating conditions and degradation pathway. Sep. Purif. Technol. 2017, 172, 158–167. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D.J. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. Int. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.Y.; Lee, B.H. Removal of Ethanolamine (ETA) and COD produced in a power plant wastewater by nano-ZVI (zerovalent iron) and hydrogen peroxide (H2O2). Int. J. Environ. Sci. 2019, 10, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Crane, R.A.; Scott, T.B.; Engineering, J.J. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- Bogacki, J.; Hazmi, H. Automotive fleet repair facility wastewater treatment using air/ZVI and air/ZVI/H2O2 processes. Arch. Environ. Prot. 2017, 43, 24–31. [Google Scholar] [CrossRef]
- Vilardi, G.; Sebastiani, D.; Miliziano, S.; Verdone, N.; Di Palma, L. Heterogeneous nZVI induced Fenton oxidation process to enhance biodegradability of excavation by products. Chem. Eng. J. 2018, 335, 309–320. [Google Scholar] [CrossRef]
- Kempthorne, O. The Design and Analysis of Experiments; American Psychological Association: Washington, DC, USA, 1952. [Google Scholar]
- Ahmad, A.; Ismail, S.; Bhatia, S. Optimization of coagulation− flocculation process for palm oil mill effluent using response surface methodology. Environ. Sci. Technol. 2005, 39, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.R.; Ibrahim, A.H. COD removal from anaerobically treated palm oil mill effluent (AT-POME) via aerated heterogeneous Fenton process: Optimization study. J. Water Process. Eng. 2014, 1, 08–16. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; El-Tayieb, M.M.; Ahmed, N.A.S.; Mostafa, A.M. Algorithms and statistics for municipal wastewater treatment using nano zero valent iron (nZVI). J. Environ. Biotechnol. 2018, 7, 30–44. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Nikazar, M. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; Volume 4. [Google Scholar]
- Taha, M.R.; Ibrahim, A.H. Characterization of nano zero-valent iron (nZVI) and its application in sono-Fenton process to remove COD in palm oil mill effluent. J. Environ. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Giasuddin, A.B.; Kanel, S.R.; Choi, H. Technology. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Elliott, D.W.; Zhang, W.X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Sun, Y.P.; Li, X.Q.; Cao, J.; Zhang, W.X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Kang, Y.G.; Yoon, H.; Lee, W.; Kim, E.J.; Chang, Y.S. Comparative study of peroxide oxidants activated by nZVI: Removal of 1-, 4-Dioxane and arsenic(III) in contaminated waters. Int. J. Chem. Eng. 2018, 334, 2511–2519. [Google Scholar] [CrossRef]
- Shafieiyoun, S.; Ebadi, T.; Nikazar, M. Treatment of landfill leachate by Fenton process with nano sized zero valent iron particles. Int. J. Environ. Sci. 2012, 6, 119–128. [Google Scholar]
- Rasool, K.; Woo, S.H.; Lee, D.S. Simultaneous removal of COD and Direct Red 80 in a mixed anaerobic sulfate-reducing bacteria culture. Chem. Eng. J. 2013, 223, 611–616. [Google Scholar] [CrossRef]
- Singa, P.K.; Isa, M.H.; Ho, Y.C.; Lim, J.W. Mineralization of Hazardous Waste Landfill Leachate Using Photo-FENTON Process. In E3S Web Conference; EDP Sciences: Les Ulis, France, 2018; p. 05012. [Google Scholar]
- Valentukeviciene, M.; Bagdziunaite, L.L.; Chadyšas, V.; Litvinaitis, A.J.S. Evaluating the impacts of integrated pollution on water quality of the trans-boundary neris (viliya) river. Sustainability 2018, 10, 4239. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Zhang, D.; Fang, J. A novel Fe(II)/citrate/UV/peroxymonosulfate process for micropollutant degradation: Optimization by response surface methodology and effects of water matrices. Chemosphere 2017, 184, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.; Malek, M.; Chong, F. Red Lentil (Lens culinaris) extract as a novel natural coagulant for turbidity reduction: An evaluation, characterization and performance optimization study. Water 2019, 11, 1686. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.; Chong, F.; Yen, C. Valorization of conventional rice starch in drinking water treatment and optimization using response surface methodology (RSM). Chem. Eng. Commun. 2019, 1–11. [Google Scholar] [CrossRef]
- Wei, W.; Han, X.; Zhang, M.; Zhang, Y.; Zheng, C.J.I.J.o.B.M. Macromolecular humic acid modified nano-hydroxyapatite for simultaneous removal of Cu (II) and methylene blue from aqueous solution: Experimental design and adsorption study. Int. J. Biol. Macromol. 2020, 150, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.F.; Muyeed, A.A.; Ahmad, T. Environmental Sanitation Wastewater Wreatment and Disposal; University Grants Commission of Bangladesh: Dhaka, Bangladesh, 2014. [Google Scholar]
- Lu, J.; Wang, X.; Shan, B.; Li, X.; Wang, W. Analysis of chemical compositions contributable to chemical oxygen demand (COD) of oilfield produced water. Chemosphere 2006, 62, 322–331. [Google Scholar] [CrossRef]
- Aljuboury, D.A.; Palaniandy, P.H.B.; Feroz, S. Treatment of petroleum wastewater by conventional and new technologies-A review. Glob. Nest J. 2017, 19, 439–452. [Google Scholar]
- Chowdhury, K.H.; Husain, T.; Veitch, B.; Hawboldt, K. Probabilistic risk assessment of polycyclic aromatic hydrocarbons (PAHs) in produced water. Hum. Ecol. Risk Assess. 2010, 15, 1049–1063. [Google Scholar] [CrossRef]
- Durell, G.S.; Johnsen, S. Determining produced water originating polycyclic aromatic hydrocarbons in North Sea waters: Comparison of sampling techniques. Mar. Pollut. Bull. 1999, 38, 977–989. [Google Scholar]
- Jagadevan, S.; Jayamurthy, M.; Dobson, P. A novel hybrid nano zerovalent iron initiated oxidation e biological degradation approach for remediation of recalcitrant waste metalworking fluids. Water Res. 2012, 46, 2395–2404. [Google Scholar] [CrossRef]
- Joo, S.H.; Feitz, A.J.; Sedlak, D.L.; Waite, T.D. Quantification of the oxidizing capacity of nanoparticulate zero-valent iron. Environ. Sci. Technol. 2005, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Manan, T.S.; Khan, T.; Sivapalan, S. Application of response surface methodology for the optimization of polycyclic aromatic hydrocarbons degradation from potable water using photo-Fenton oxidation process. Sci. Total Environ. 2019, 665, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jin, X.; Kuang, Y.; Megharaj, M.; Naidu, R.; Chen, Z. An integrated biodegradation and nano oxidation used for the remediation of naphthalene from aqueous solution. Chemosphere 2015, 141, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Palma, L.D.; Verdone, N. On the critical use of zero valent iron nanoparticles and Fenton processes for the treatment of tannery wastewater. J. Water Process. Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Boussahel, R. Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard. Mater. 2009, 163, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Wijesekaras, H.; Basnayake, A.; Vithanage, M. Organic-coated nanoparticulate zero valent iron for remediation of chemical oxygen demand (COD) and dissolved metals from tropical landfill leachate. Environ. Sci. Pollut. Res. 2014, 21, 7075–7087. [Google Scholar] [CrossRef]
- Pardo, F.; Peluffo, M.; Santos, A. Optimization of the application of the Fenton chemistry for the remediation of a contaminated soil with polycyclic aromatic hydrocarbons. J. Chem. Technol. Biot. 2016, 91, 1763–1772. [Google Scholar] [CrossRef]
- Karimi, B.; Rajaei, M.; Koulivand, A.; Cheshmeh, R. Performance evaluation of advanced Fe°/Fe+2/Fe+3/H2O2 process in the reduction of nitrate and organic matter from aqueous solution. Desalin. Water Treat. 2014, 52, 6240–6248. [Google Scholar] [CrossRef]
- Borror, C.M.; Montgomery, D.C. Response surface design evaluation and comparison. J. Stat. Plan. Inference 2009, 139, 629–641. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, S.T.; Separation, J.; Technology, P. Performance of nZVI/H2O2 process in degrading polyvinyl alcohol in aqueous solutions. Sep. Purif. Technol. 2018, 203, 111–116. [Google Scholar] [CrossRef]
- Cheng, R.; Cheng, C.; Liu, G.H.; Zheng, X.; Li, G.; Li, J.J.C. Removing pentachlorophenol from water using a nanoscale zero-valent iron/H2O2 system. Chemosphere 2015, 141, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Rodríguez-Rodríguez, J.; Ochando-Pulido, J.M.; Verdone, N.; Martinez-Ferez, A.; Di Palma, L.; Protection, E. Large Laboratory-Plant application for the treatment of a Tannery wastewater by Fenton oxidation: Fe (II) and nZVI catalysts comparison and kinetic modelling. Process. Saf. Environ. Prot. 2018, 117, 629–638. [Google Scholar] [CrossRef]
- Ahmadi, M.; Rahmani, K.; Rahmani, A.; Rahmani, H.J. Removal of benzotriazole by Photo-Fenton like process using nano zero-valent iron: Response surface methodology with a Box-Behnken design. Pol. J. Chem. Technol. 2017, 19, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [Green Version]
- Ertugay, N.; Kocakaplan, N.; Malkoç, E. Investigation of pH effect by Fenton-like oxidation with ZVI in treatment of the landfill leachate. Int. J. Min. Reclam. Env. 2017, 31, 404–411. [Google Scholar] [CrossRef]
- Usman, M.; Cheema, S.A.; Farooq, M.J. Heterogeneous Fenton and persulfate oxidation for treatment of landfill leachate: A review supplement. J. Clean. Prod. 2020, 256, 120448. [Google Scholar] [CrossRef]
- Shi, X.Y.; Jin, D.W.; Sun, Q.Y.; Li, W.W. Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew. Energy 2010, 35, 1493–1498. [Google Scholar] [CrossRef]


| Factors | Independent Variables | Coded Values | Star Point α = 1 | |||
|---|---|---|---|---|---|---|
| −1 | 0 | 1 | −α | +α | ||
| A | nZVI conc. (g/L) | 1.0 | 3.50 | 6.0 | 1.0 | 6.0 |
| B | Hydrogen peroxide (g/L) | 0.4 | 1.625 | 2.85 | 0.4 | 2.85 |
| C | Contact time (min) | 30 | 115 | 200 | 30 | 200 |
| D | pH | 1.0 | 3.0 | 5.0 | 1.0 | 5.0 |
| Experiment No. | Independent Variables | |||
|---|---|---|---|---|
| nZVI Conc. (g/L) | H2O2 Conc. (g/L) | Contact Time (min) | pH | |
| 1 | 6.0 | 2.85 | 200 | 1.0 |
| 2 | 6.0 | 0.40 | 30 | 1.0 |
| 3 | 3.5 | 1.62 | 115 | 3.0 |
| 4 | 3.5 | 2.85 | 115 | 3.0 |
| 5 | 3.5 | 1.62 | 115 | 3.0 |
| 6 | 3.5 | 1.62 | 115 | 3.0 |
| 7 | 1.0 | 2.85 | 200 | 1.0 |
| 8 | 3.5 | 1.62 | 115 | 3.0 |
| 9 | 3.5 | 0.40 | 115 | 3.0 |
| 10 | 3.5 | 1.62 | 200 | 3.0 |
| 11 | 1.0 | 0.40 | 200 | 1.0 |
| 12 | 6.0 | 1.62 | 115 | 3.0 |
| 13 | 1.0 | 2.85 | 30 | 1.0 |
| 14 | 1.0 | 2.85 | 30 | 5.0 |
| 15 | 3.5 | 1.62 | 115 | 1.0 |
| 16 | 6.0 | 2.85 | 30 | 5.0 |
| 17 | 3.5 | 1.62 | 30 | 3.0 |
| 18 | 1.0 | 0.40 | 30 | 5.0 |
| 19 | 6.0 | 0.40 | 30 | 5.0 |
| 20 | 3.5 | 1.62 | 115 | 3.0 |
| 21 | 3.5 | 1.62 | 115 | 5.0 |
| 22 | 6.0 | 2.85 | 200 | 5.0 |
| 23 | 1.0 | 0.40 | 200 | 5.0 |
| 24 | 6.0 | 2.85 | 30 | 1.0 |
| 25 | 1.0 | 1.62 | 115 | 3.0 |
| 26 | 3.5 | 1.62 | 115 | 3.0 |
| 27 | 1.0 | 2.85 | 200 | 5.0 |
| 28 | 6.0 | 0.40 | 200 | 5.0 |
| 29 | 1.0 | 0.40 | 30 | 1.0 |
| 30 | 6.0 | 0.40 | 200 | 1.0 |
| Characteristics | Concentration (mg/L) |
|---|---|
| BOD | 50.0 |
| COD | 2213.0 |
| TSS | 139 |
| Turbidity | 85 NTU |
| NH3-N | 28.0 |
| TOC | 759.4 |
| Method | Type of Effluents | Pollutants | Removal (%) | References |
|---|---|---|---|---|
| nZVI/H2O2 | Synthetic wastewater | Polyvinyl alcohol | 94.0 | [55] |
| nZVI/H2O2 | Synthetic wastewater | pentachlorophenol | 57.0 | [56] |
| nZVI/H2O2 | Tannery wastewater | COD | 75.5 | [57] |
| nZVI/H2O2 | Synthetic wastewater | Benzotriazole and COD | 73.4 and 40.0 | [58] |
| nZVI/H2O2 | Synthetic wastewater | Amoxicillin and COD | 86.5 and 71.2 | [11] |
| nZVI/H2O2 | Contaminated soil | PAHs | 80.0 | [52] |
| nZVI/H2O2 | Tannery wastewater | TOC, COD and phenol | 70.0, 73.0 and 88.0 | [59] |
| nZVI/H2O2 | Landfill Leachate | COD | 74.0 | [60] |
| nZVI/H2O2/microwave irradiation | Landfill Leachate | Organic compounds | 58.0 | [61] |
| nZVI/H2O2/biodegradation | Synthetic wastewater | COD and naphthalene | 55.2 and 99.0 | [47] |
| nZVI/H2O2 | Produced water | COD and PAHs | 76.3 and 89.5 | This Study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst. Water 2020, 12, 2430. https://0-doi-org.brum.beds.ac.uk/10.3390/w12092430
Haneef T, Ul Mustafa MR, Rasool K, Ho YC, Mohamed Kutty SR. Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst. Water. 2020; 12(9):2430. https://0-doi-org.brum.beds.ac.uk/10.3390/w12092430
Chicago/Turabian StyleHaneef, Tahir, Muhammad Raza Ul Mustafa, Kashif Rasool, Yeek Chia Ho, and Shamsul Rahman Mohamed Kutty. 2020. "Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst" Water 12, no. 9: 2430. https://0-doi-org.brum.beds.ac.uk/10.3390/w12092430






