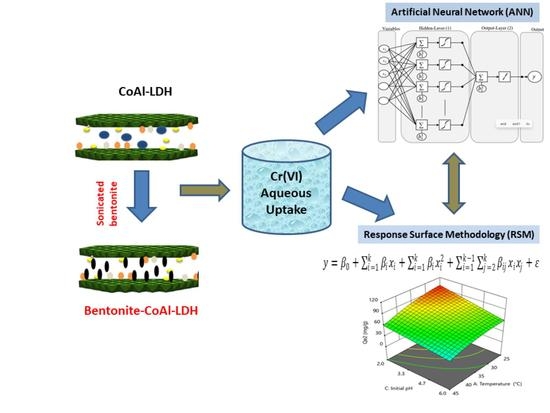
Insight into ANN and RSM Models’ Predictive Performance for Mechanistic Aspects of Cr(VI) Uptake by Layered Double Hydroxide Nanocomposites from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Synthesis, and Characterizations of LDHs
2.2. Characterization and Analysis Methods
2.3. Cr(VI) Adsorption onto CoAl-LDH and Bentonite-CoAl from Aqueous Phase Tests
2.3.1. FC-CCD Study
2.3.2. Kinetics and Thermodynamics Study
2.4. Math Techniques and Performance Evaluation
2.4.1. RSM Experiments and Modeling
2.4.2. Artificial Neural Network (ANN) Models Development
2.4.3. Developed Models’ Performance Evaluation
3. Results and Discussion
3.1. Adsorbent Characterization Analysis
3.2. Cr(VI) RSM Sorption Model Development and Evaluation
3.2.1. Cr(VI) Sorption RSM Model Development
3.2.2. Cr(VI) Adsorption Pareto Charts
3.2.3. Cr(VI) Adsorption RSM Model Validation
3.3. Cr(VI) Uptake Capacity Operational Parameter Influence and Optimization
3.4. Cr(VI) ANN Uptake Modeling and Optimization Strategy
3.5. ANN vs. RSM Cr(VI) Adsorption Models’ Comparative Performance
3.5.1. ANN and RSM Prediction of FC-CCD Data for LDHs Cr(VI) Uptake
3.5.2. ANN and RSM Prediction of Equilibrium and Thermodynamics of Cr(VI) Uptake
3.5.3. ANN Prediction of Kinetic of Cr(VI) Uptake
4. Conclusions
- The Cr(VI) uptake capacity data obtained for the adsorbents effectively fits the quartic RSM polynomial models (R2 = 0.997) with insignificant lack of fit (p-value < 0.05).
- The Cr (VI) uptake capacity improved with increasing Cr(VI) initial concentration and initial pH, while increasing the operational temperature with optimal conditions obtained at temperature 25 °C, pH = 2 and 126 mg/L initial Cr(VI) concentration.
- Levenberg-Marquardt ANN algorithms (ANN-LMA) converged faster and better compared to other tested ANN-based algorithms.
- Both the RSM and ANN-LMA models performed well and based on the non-linear Langmuir model KL values, they predicted −ΔG°, −ΔH and −ΔS which supported the actual feasibility, spontaneity and greater order of reaction as well as the exothermic nature of Cr(VI) uptake onto the tested adsorbents.
- The ANN-LMA models’ accurate kinetic parameter predictions further indicated a mainly pseudo-second-order process conforming the predominant chemisorption mechanism, which are well established by the Cr(VI) speciation and surface charges for the Cr(VI) uptake by both CoAl-LDH and bentonite-CoAl-LDH.
- The ANN-LMA models’ predictions were better compared to the RSM predictions, and the non-linearized forms of the kinetics and equilibrium models provided better parameters compared to the linearized forms.
- The ANN-LMA models indicated a consistent and insignificant decline in their prediction potentials under the different mechanistic studies undertaken.
- This study demonstrates the high potential reliability of RSM and ANN-LMA models in capturing Cr(VI) adsorption data for LDHs nanocomposites heavy metal uptake in water and wastewater treatment.
- It is recommended to undertake further studies on the influence of independent variable analysis and sensitivity analysis for the RSM and ANN-LMA models on equilibrium, kinetic, and thermodynamic predictability to further establish their potentials.
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynel-Ávila, H.E.; Aguayo-Villarreal, I.A.; Diaz-Muñoz, L.L.; Moreno-Pérez, J.; Sánchez-Ruiz, F.J.; Rojas-Mayorga, C.K.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A Review of the Modeling of Adsorption of Organic and Inorganic Pollutants from Water Using Artificial Neural Networks. Adsorpt. Sci. Technol. 2022, 2022, 9384871. [Google Scholar] [CrossRef]
- Haladu, S.A.; Dalhat Mu’azu, N.; Ali, S.A.; Elsharif, A.M.; Odewunmi, N.A.; Abd El-Lateef, H.M. Inhibition of mild steel corrosion in 1 M H2SO4 by a gemini surfactant 1,6-hexyldiyl-bis-(dimethyldodecylammonium bromide): ANN, RSM predictive modeling, quantum chemical and MD simulation studies. J. Mol. Liq. 2022, 350, 118533. [Google Scholar] [CrossRef]
- Yaseen, Z.M. An insight into machine learning models era in simulating soil, water bodies and adsorption heavy metals: Review, challenges and solutions. Chemosphere 2021, 277, 130126. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.O.; Akinlabi, S.; Madushele, N.; Adedeji, P.A. Property-based biomass feedstock grading using k-Nearest Neighbour technique. Energy 2020, 190, 116346. [Google Scholar] [CrossRef]
- Syah, R.; Piri, F.; Elveny, M.; Khan, A. Artificial Intelligence simulation of water treatment using nanostructure composite ordered materials. J. Mol. Liq. 2021, 345, 117046. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: New York, NY, USA, 2016; ISBN 1118916034. [Google Scholar]
- Karaman, C.; Karaman, O.; Show, P.-L.; Karimi-Maleh, H.; Zare, N. Congo red dye removal from aqueous environment by cationic surfactant modified-biomass derived carbon: Equilibrium, kinetic, and thermodynamic modeling, and forecasting via artificial neural network approach. Chemosphere 2022, 290, 133346. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M. A comparison of ann and rsm models for anionic dye adsorption onto bentonite-clay intercalated cobalt-aluminum ldh nanocomposites. Desalin. Water Treat. 2020, 179, 340–353. [Google Scholar] [CrossRef]
- Fetimi, A.; Dâas, A.; Benguerba, Y.; Merouani, S.; Hamachi, M.; Kebiche-Senhadji, O.; Hamdaoui, O. Optimization and prediction of safranin-O cationic dye removal from aqueous solution by emulsion liquid membrane (ELM) using artificial neural network-particle swarm optimization (ANN-PSO) hybrid model and response surface methodology (RSM). J. Environ. Chem. Eng. 2021, 9, 105837. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Afolabi, H.K.; Olalere, O.A. Efficient extraction of antioxidants from Vernonia cinerea leaves: Comparing response surface methodology and artificial neural network. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 276–285. [Google Scholar] [CrossRef]
- Dalhat, M.A.; Mu’Azu, N.D.; Essa, M.H. Generalized decay and artificial neural network models for fixed-Bed phenolic compounds adsorption onto activated date palm biochar. J. Environ. Chem. Eng. 2021, 9, 104711. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P. Heavy metal contamination in water and its possible sources. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–189. [Google Scholar]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of nanomaterials for heavy metal removal from water and soil: A review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Jarrah, N.; Mu’azu, N.D.; Zubair, M.; Al-Harthi, M. Enhanced adsorptive performance of Cr(VI) onto layered double hydroxide-bentonite composite: Isotherm, kinetic and thermodynamic studies. Sep. Sci. Technol. 2020, 55, 1897–1909. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, C.; Zheng, J.; Yu, H.; Chen, Q.; Guo, L.; Pan, D.; Naik, N.; Shao, Q.; Guo, Z. Microwave hydrothermal fabrication of CuFeCr ternary layered double hydroxides with excellent Cr(VI) adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127279. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Y. Ultrafast advanced treatment of chromium complex-containing wastewater using Co/Fe layered double hydroxide. Environ. Technol. Innov. 2022, 26, 102296. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, X.; Zhang, Y.-X.; Liu, Z.-H. Feasible synthesis of hierarchical porous MgAl-borate LDHs functionalized Fe3O4@SiO2 magnetic microspheres with excellent adsorption performance toward congo red and Cr(VI) pollutants. J. Alloys Compd. 2021, 861, 157974. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Zheng, J.; Shang, L.; Shi, Y.; Wu, Q.; Liu, X.; Wang, Y.; Shi, L.; Shao, Q. Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI). Adv. Compos. Hybrid Mater. 2021, 4, 819–829. [Google Scholar] [CrossRef]
- Manea, Y.K.; Khan, A.M.; Wani, A.A.; Saleh, M.A.S.; Qashqoosh, M.T.A.; Shahadat, M.; Rezakazemi, M. In-grown flower like Al-Li/Th-LDH@CNT nanocomposite for enhanced photocatalytic degradation of MG dye and selective adsorption of Cr (VI). J. Environ. Chem. Eng. 2022, 10, 106848. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Liu, K.; Zhou, S.; Alqsair, U.F.; El-Shafay, A.S. Machine learning simulation of Cr (VI) separation from aqueous solutions via a hierarchical nanostructure material. J. Mol. Liq. 2022, 350, 118565. [Google Scholar] [CrossRef]
- Ait-Amir, B.; Pougnet, P.; El Hami, A. Meta-Model Development. In Embedded Mechatronic Systems 2; El Hami, A., Pougnet, P.B.T.-E.M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–187. ISBN 978-1-78548-190-1. [Google Scholar]
- Elkhider, K.H.A.; Ihsanullah, I.; Zubair, M.; Manzar, M.S.; Mu’azu, N.D.; Al-Harthi, M.A. Synthesis, Characterization and Dye Adsorption Performance of Strontium Ferrite decorated Bentonite-CoNiAl Magnetic Composite. Arab. J. Sci. Eng. 2020, 45, 7397–7408. [Google Scholar] [CrossRef]
- Blaisi, N.I.; Zubair, M. Date palm ash-MgAl-layered double hydroxide composite: Sustainable adsorbent for effective removal of methyl orange and eriochrome black-T from aqueous phase. Environ. Sci. Pollut. Res. 2018, 25, 34319–34331. [Google Scholar] [CrossRef]
- Waheed, A.; Kazi, I.W.; Manzar, M.S.; Ahmad, T.; Mansha, M.; Ullah, N.; Ahmed Blaisi, N.I. Ultrahigh and efficient removal of Methyl orange, Eriochrom Black T and acid Blue 92 by triazine based cross-linked polyamine resin: Synthesis, isotherm and kinetic studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125472. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, Y.; Yang, K.; Yu, S.; Yu, H.; Zhu, B.; Du, B. Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Wan, Y. Adsorption and Reduction of Aqueous Cr by FeS-Modified Fe-Al Layered Double Hydroxide. Sustainability 2021, 14, 21. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Sheng, G.; Hu, J.; Li, H.; Li, J.; Huang, Y. Enhanced sequestration of Cr(VI) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study. Chemosphere 2016, 148, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; He, X.; Xiao, M.; Zhang, W.; Lu, C. A super biosorbent from dendrimer poly (amidoamine)-grafted cellulose nanofibril aerogels for effective removal of Cr (VI). J. Mater. Chem. A 2015, 3, 14703–14711. [Google Scholar] [CrossRef]
- Naja, G.; Volesky, B. The Mechanism of Metal Cation and Anion Biosorption. In Microbial Biosorption of Metals; Kotrba, P., Mackova, M., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 19–58. ISBN 978-94-007-0443-5. [Google Scholar]
- Yadav, A.M.; Nikkam, S.; Gajbhiye, P.; Tyeb, M.H. Modeling and optimization of coal oil agglomeration using response surface methodology and artificial neural network approaches. Int. J. Miner. Process. 2017, 163, 55–63. [Google Scholar] [CrossRef]
- Khamparia, A.; Pandey, B.; Pandey, D.K.; Gupta, D.; Khanna, A.; de Albuquerque, V.H.C. Comparison of RSM, ANN and Fuzzy Logic for extraction of Oleonolic Acid from Ocimum sanctum. Comput. Ind. 2020, 117, 103200. [Google Scholar] [CrossRef]
- Ray, S.; Haque, M.; Ahmed, T.; Nahin, T.T. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in predicting the compressive and splitting tensile strength of concrete prepared with glass waste and tin (Sn) can fiber. J. King Saud Univ.-Eng. Sci. 2021. [Google Scholar] [CrossRef]
- Nuapia, Y.; Cukrowska, E.; Tutu, H.; Chimuka, L. Statistical comparison of two modeling methods on pressurized hot water extraction of vitamin C and phenolic compounds from Moringa oleifera leaves. S. Afr. J. Bot. 2018, 129, 9–16. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Manzar, M.S.; Kazeem, T.S.; Qureshi, A.; Haladu, S.A.; Blaisi, N.I.; Essa, M.H.; Al-Harthi, M.A. Mechanistic aspects of magnetic MgAlNi barium-ferrite nanocomposites enhanced adsorptive removal of an anionic dye from aqueous phase. J. Saudi Chem. Soc. 2020, 24, 715–732. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zhang, S.; Yu, Q.; Cheng, J.; Qiu, X. Ultrasound-assisted synthesis of single layer MgAl hydrotalcite for the removal of Cr(VI) in solution and soil. Appl. Clay Sci. 2021, 204, 106025. [Google Scholar] [CrossRef]
- Babu Poudel, M.; Shin, M.; Joo Kim, H. Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr(VI), Pb(II), and As(III) ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Wani, A.A.; Khan, A.M.; Manea, Y.K.; Salem, M.A.S.; Shahadat, M. Selective adsorption and ultrafast fluorescent detection of Cr(VI) in wastewater using neodymium doped polyaniline supported layered double hydroxide nanocomposite. J. Hazard. Mater. 2021, 416, 125754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhang, Q.; Wang, W.; Yang, C.; Du, T.; Yue, T.; Zhu, M.; Wang, J. Demand-oriented construction of Mo3S13-LDH: A versatile scavenger for highly selective and efficient removal of toxic Ag(I), Hg(II), As(III), and Cr(VI) from water. Sci. Total Environ. 2022, 820, 153334. [Google Scholar] [CrossRef] [PubMed]
- Rezak, N.; Bahmani, A.; Bettahar, N. Adsorptive removal of P(V) and Cr(VI) by calcined Zn-Al-Fe ternary LDHs. Water Sci. Technol. 2021, 83, 2504–2517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dai, T.; Qiao, Z.; Sun, P.; Hao, J.; Yang, Y. Application of artificial intelligence to wastewater treatment: A bibliometric analysis and systematic review of technology, economy, management, and wastewater reuse. Process Saf. Environ. Prot. 2020, 133, 169–182. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Vafaei, A. Applications of artificial neural networks for adsorption removal of dyes from aqueous solution: A review. Adv. Colloid Interface Sci. 2017, 245, 20–39. [Google Scholar] [CrossRef]
- Nag, S.; Bar, N.; Das, S.K. Sustainable bioremadiation of Cd(II) in fixed bed column using green adsorbents: Application of Kinetic models and GA-ANN technique. Environ. Technol. Innov. 2019, 13, 130–145. [Google Scholar] [CrossRef]












| Experimental Variables | Levels | |||
|---|---|---|---|---|
| High-Lower (+1) | Mid-Level (0) | Lower Level (−1) | ||
| A | Temperature (°C) | 45 | 35 | 25 |
| B | Initial Cr(IV) conc. (mg/L) | 127 | 76.5 | 26 |
| C | Initial pH | 6 | 4 | 2 |
| Standard Order | Run Order | A: Temperature °C | B: Inital Cr(VI) Conc. mg/L | C: Initial pH | Qe1, mg/g | Qe2 mg/g |
|---|---|---|---|---|---|---|
| 16 | 1 | 35 | 76.5 | 4 | 38.10 | 51.36 |
| 4 | 2 | 45 | 127 | 2 | 58.32 | 63.92 |
| 3 | 3 | 25 | 127 | 2 | 77.44 | 101.36 |
| 2 | 4 | 45 | 26 | 2 | 18.80 | 20.4 |
| 14 | 5 | 35 | 76.5 | 6 | 43.20 | 43.44 |
| 7 | 6 | 25 | 127 | 6 | 43.60 | 55.76 |
| 17 | 7 | 35 | 76.5 | 4 | 36.99 | 53.212 |
| 8 | 8 | 45 | 127 | 6 | 36.40 | 45.76 |
| 15 | 9 | 35 | 76.5 | 4 | 37.44 | 52.48 |
| 11 | 10 | 35 | 26 | 4 | 19.60 | 25.84 |
| 13 | 11 | 35 | 76.5 | 2 | 45.44 | 58.4 |
| 12 | 12 | 35 | 127 | 4 | 44.48 | 75.12 |
| 1 | 13 | 25 | 26 | 2 | 35.92 | 34.8 |
| 5 | 14 | 25 | 26 | 6 | 19.12 | 19.84 |
| 10 | 15 | 45 | 76.5 | 4 | 76.88 | 30.64 |
| 9 | 16 | 25 | 76.5 | 4 | 63.04 | 71.44 |
| 6 | 17 | 45 | 26 | 6 | 19.68 | 15.52 |
| Raw Bentonite | CoAl-LDH | Bentonite-CoAl-LDH | |||
|---|---|---|---|---|---|
| Element | Weight % | Element | Weight % | Element | Weight % |
| C | 13.87 | O | 24.69 | C | 8.68 |
| O | 51.83 | Al | 5.72 | O | 39.12 |
| Na | 1.16 | Co | 69.59 | Al | 6.54 |
| Mg | 1.33 | - | - | Si | 2.64 |
| Al | 7.47 | Co | 40.36 | ||
| Si | 18.22 | - | - | Cu | 2.66 |
| Cl | 1.06 | - | - | ||
| Ca | 0.56 | - | - | ||
| Fe | 2.66 | - | - | ||
| Cu | 1.85 | - | - | ||
| Totals | 100.00 | - | 100 | - | 100 |
| Qe1 | Qe2 | ||||
|---|---|---|---|---|---|
| Source | F-Value | p-Value | Source | F-Value | p-Value |
| Model | 1236.10 | 0.0008 | Model | 50.82 | 0.0009 |
| A | 307.26 | 0.0032 | A | 62.20 | 0.0014 |
| B | 992.97 | 0.0010 | B | 90.74 | 0.0007 |
| C | 8.05 | 0.1050 | C | 72.60 | 0.0010 |
| AB | 38.20 | 0.0252 | AB | 7.71 | 0.0500 |
| AC | 351.36 | 0.0028 | AC | 13.15 | 0.0222 |
| BC | 636.52 | 0.0016 | BC | 18.02 | 0.0132 |
| A2 | 4053.91 | 0.0002 | A2 | 0.1541 | 0.7147 |
| B2 | 115.19 | 0.0086 | B2 | 0.3138 | 0.6052 |
| C2 | 178.54 | 0.0056 | C2 | 0.1836 | 0.6904 |
| ABC | 13.31 | 0.0676 | A2B | 0.8145 | 0.4178 |
| A2B | 41.40 | 0.0233 | AB2 | 17.59 | 0.0138 |
| A2C | 315.51 | 0.0032 | A2B2 | 0.2379 | 0.6513 |
| AB2 | 774.07 | 0.0013 | |||
| A2B2 | 1155.36 | 0.0009 | |||
| CoAl-LDH (Qe1) | Bentonite-CoAl-LDH (Qe2) | |||||
|---|---|---|---|---|---|---|
| Run Order | Actual Value | Predicted Value | Residual | Actual Value | Predicted Value | Residual |
| 1 | 38.10 | 37.51 | 0.5900 | 51.36 | 52.35 | −0.9907 |
| 2 | 58.32 | 58.32 | 0.0000 | 63.92 | 65.50 | −1.58 |
| 3 | 77.44 | 77.44 | 0.0000 | 101.36 | 98.60 | 2.76 |
| 4 | 18.80 | 18.80 | 0.0000 | 20.40 | 17.64 | 2.76 |
| 5 | 43.20 | 43.20 | 0.0000 | 43.44 | 41.06 | 2.38 |
| 6 | 43.60 | 43.60 | 0.0000 | 55.76 | 58.52 | −2.76 |
| 7 | 36.99 | 37.51 | −0.5200 | 53.21 | 52.35 | 0.8613 |
| 8 | 36.40 | 36.40 | 0.0000 | 45.76 | 44.18 | 1.58 |
| 9 | 37.44 | 37.51 | −0.0700 | 52.48 | 52.35 | 0.1293 |
| 10 | 19.60 | 19.60 | 0.0000 | 25.84 | 25.84 | 0.0000 |
| 11 | 45.44 | 45.44 | 0.0000 | 58.40 | 60.78 | −2.38 |
| 12 | 44.48 | 44.48 | 0.0000 | 75.12 | 75.12 | 0.0000 |
| 13 | 35.92 | 35.92 | 0.0000 | 34.80 | 36.38 | −1.58 |
| 14 | 19.12 | 19.12 | 0.0000 | 19.84 | 18.26 | 1.58 |
| 15 | 76.88 | 76.88 | 0.0000 | 30.64 | 30.64 | 0.0000 |
| 16 | 63.04 | 63.04 | 0.0000 | 71.44 | 71.44 | 0.0000 |
| 17 | 19.68 | 19.68 | 0.0000 | 15.52 | 18.28 | −2.76 |
| Model | Parameter | Qe1 | Qe2 | |
|---|---|---|---|---|
| FC-CCD study | RSM | R2 | 0.999 | 0.997 |
| RMSE | 0.195 | 1.774 | ||
| ANN-LMA | No. of neurons | 5 | 10 | |
| Epoch | 6 | 5 | ||
| Training R2 | 0.999 | 0.999 | ||
| Validation R2 | 0.998 | 0.999 | ||
| Testing R2 | 0.903 | 0.999 | ||
| Overall R2 | 0.995 | 0.997 | ||
| RMSE | 3.092 | 2.982 | ||
|
Cr(VI) uptake Equilibrium study | RSM | R2 | 0.8715 | 0.9793 |
| RMSE | 20.50 | 8.00 | ||
| ANN-LMA | No. of neurons | 7 | 10 | |
| Epoch | 10 | 4 | ||
| Training R2 | 0.998 | 0.999 | ||
| Validation R2 | 0.905 | 0.966 | ||
| Testing R2 | 0.995 | 0.982 | ||
| Overall R2 | 0.993 | 0.992 | ||
| RMSE | 4.089 | 4.99 | ||
|
Cr(VI) uptake Kinetics study | ANN-LMA | No. of neurons | 10 | 10 |
| Epoch | 4 | 17 | ||
| Training R2 | 0.996 | 0.999 | ||
| Validation R2 | 0.999 | 0.998 | ||
| Testing R2 | 0.995 | 0.995 | ||
| Overall R2 | 0.999 | 0.998 | ||
| RMSE | 17.32 | 6.75 |
| # | LDH | qmax (mg/g) | Model’s Fittings | Reference | |
|---|---|---|---|---|---|
| Isotherm | Kinetics | ||||
| 1 | CoAl-LDH | 121.1 | Langmuir | Pseudo-second-order | This study |
| 2 | Bentonite-CoAl-LDH | 205.2 | Langmuir | Pseudo-second-order | This study |
| 3 | CuFeCr-LDH | 22.24 | Langmuir | Pseudo-second-order | [20] |
| 4 | F-U-MgAl-LDH | 7.26 | Freundlich | Pseudo-second-order | [43] |
| 5 | Co2Fe1-CO3-LDH | 5.44 | Langmuir and Freundlich | Pseudo-second-order | [21] |
| FeS modified FeAl-LDH | 147.7 | Langmuir | [32] | ||
| 7 | Co-Al-LDH@Fe2O3/3DPCNF | 400.40 | Sips | [33] | |
| 8 | MnFe-LDH/MnFe2O3@3DNF | 564.88 | Langmuir, Freundlich and Sips | Pseudo-second-order | [44] |
| 9 | Fe3O4@SiO2@MgAl-borate LDH | 86.73 | Langmuir | Pseudo-second-order | [22] |
| 10 | ZnNiCr-LDH | 28.2 | Langmuir | Pseudo-second-order | [23] |
| 11 | PANI@ZNd-ZnAl-LDH | 219 | Langmuir | Pseudo-second-order | [45] |
| 12 | Mo3S13-MgAl-LDH | 90.6 | [46] | ||
| 13 | Al-Li/Th-LDH@CNT | 172.4 | [24] | ||
| 14 | Zn-Al-Fe-LDH | 52.63 | Langmuir | Pseudo-second-order | [47] |
| Parameter | CoAl-LDH | Bentonite-CoAl-LDH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Mathematical Representation | Temp | Exp | ANN | RSM | Exp | ANN | RSM | |
| Langmuir | 25 °C | R2 | 0.9540 | 0.9915 | 0.9937 | 0.9665 | 0.9608 | 0.9937 | |
| RMSE | 6.9909 | 2.1665 | 2.9127 | 7.6944 | 8.5455 | 2.9127 | |||
| qmax, mg/g | 121.1374 | 107.1379 | 173.0420 | 197.1809 | 199.2454 | 173.0420 | |||
| KL, L/mg | 0.0215 | 0.0228 | 0.0127 | 0.0104 | 0.0099 | 0.0127 | |||
| Freundlich | R2 | 0.9164 | 0.9940 | 0.9770 | 0.9498 | 0.9407 | 0.9770 | ||
| RMSE | 9.2974 | 2.4131 | 5.3478 | 9.4950 | 10.4106 | 5.3478 | |||
| KF | 9.1121 | 8.9650 | 5.9300 | 4.9316 | 4.7647 | 5.9300 | |||
| 1/n | 0.4777 | 0.4611 | 0.6073 | 0.6558 | 0.6601 | 0.6073 | |||
| Langmuir | 35 °C | R2 | 0.9975 | 0.9879 | 0.9988 | 0.9556 | 0.9785 | 0.9882 | |
| RMSE | 1.3884 | 1.7969 | 0.5705 | 8.5942 | 3.6328 | 2.7187 | |||
| qmax, mg/g | 99.2234 | 103.1155 | 91.4632 | 168.8715 | 183.0309 | 172.9810 | |||
| KL, L/mg | 0.0117 | 0.0107 | 0.0137 | 0.0074 | 0.0067 | 0.0082 | |||
| Freundlich | R2 | 0.9945 | 0.9914 | 0.9978 | 0.9464 | 0.9781 | 0.9981 | ||
| RMSE | 1.3988 | 1.8627 | 1.0008 | 8.2754 | 4.3301 | 1.1371 | |||
| KF | 3.2380 | 3.0059 | 3.7754 | 2.7190 | 2.6078 | 3.3154 | |||
| 1/n | 0.6093 | 0.6247 | 0.5737 | 0.7118 | 0.7243 | 0.6860 | |||
| Langmuir | 45 °C | R2 | 0.9732 | 0.9864 | 0.5030 | 0.8683 | 0.9366 | 0.9110 | |
| RMSE | 3.3437 | 1.6923 | 22.3500 | 9.4967 | 6.3515 | 4.7371 | |||
| qmax, mg/g | 149.7940 | 123.1122 | 157.0474 | 205.1537 | 172.3274 | 229.0193 | |||
| KL, L/mg | 0.0058 | 0.0071 | 0.0074 | 0.0039 | 0.0056 | 0.0028 | |||
| Freundlich | R2 | 0.9773 | 0.9913 | 0.4356 | 0.8870 | 0.9519 | 0.9329 | ||
| RMSE | 3.5550 | 1.6221 | 24.1229 | 9.6128 | 6.1043 | 4.7517 | |||
| KF | 1.7519 | 1.9420 | 2.2258 | 1.5687 | 1.9947 | 1.1804 | |||
| 1/n | 0.7467 | 0.7089 | 0.7360 | 0.7812 | 0.7441 | 0.8084 | |||
| Adsorbent | Temp | ΔG (kJ/mol) | Kd (L/mmol) | ΔH (kJ/mol) | ΔS (kJ/mol K) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual | ANN | RSM | Actual | ANN | RSM | Actual | ANN | RSM | Actual | ANN | RSM | ||
| CoAl-LDH | 25 °C | −17.386 | −17.541 | −16.082 | 0.0215 | 0.0228 | 0.0127 | −51.815 | −46.421 | −21.073 | −0.115 | −0.097 | −0.016 |
| 35 °C | −16.410 | −16.196 | −16.827 | 0.0117 | 0.0107 | 0.0137 | |||||||
| 45 °C | −15.071 | −15.611 | −15.726 | 0.0058 | 0.0071 | 0.0074 | |||||||
| Bentonite-CoAl-LDH | 25 °C | −15.584 | −15.480 | −16.082 | 0.0104 | 0.0099 | 0.0127 | −38.041 | −22.988 | −59.576 | −0.075 | −0.025 | −0.145 |
| 35 °C | −15.254 | −14.982 | −15.500 | 0.0074 | 0.0067 | 0.0082 | |||||||
| 45 °C | −14.067 | −14.982 | −13.143 | 0.0039 | 0.0056 | 0.0028 | |||||||
| Model | Mathematical Representation | Initial Concentration | CoAl-LDH | Bentonite-CoAl-LDH | |||
|---|---|---|---|---|---|---|---|
| Parameter | Exp | ANN-LMA | Exp | ANN-LMA | |||
| Pseudo-first-order | ) | 20 | R2 | 0.915 | 0.946 | 0.968 | 0.997 |
| RMSE | 2.260 | 2.383 | 3.079 | 0.858 | |||
| qe | 19.741 | 20.130 | 34.273 | 20.191 | |||
| k1 | 0.112 | 0.089 | 0.060 | 2.541 | |||
| 60 | R2 | 0.909 | 0.797 | 0.830 | 0.995 | ||
| RMSE | 3.020 | 3.714 | 5.404 | 15.266 | |||
| qe | 54.290 | 52.530 | 75.312 | 62.394 | |||
| k1 | 0.0131 | 0.0143 | 0.0229 | 1.7765 | |||
| 100 | R2 | 0.996 | 0.997 | 0.959 | 0.944 | ||
| RMSE | 1.636 | 1.722 | 10.479 | 10.748 | |||
| qe | 78.458 | 80.353 | 89.023 | 104.268 | |||
| k1 | 0.080 | 0.079 | 0.139 | 0.094 | |||
| Pseudo-second-order | 20 | i2 | 0.966 | 0.985 | 0.997 | 0.997 | |
| RMSE | 1.368 | 1.519 | 1.936 | 0.860 | |||
| qe | 21.596 | 21.992 | 38.333 | 20.187 | |||
| k2 | 0.008 | 0.006 | 0.002 | 149.352 | |||
| 60 | R2 | 0.936 | 0.841 | 0.885 | 0.995 | ||
| RMSE | 2.947 | 3.598 | 4.109 | 15.268 | |||
| qe | 74.174 | 68.898 | 92.254 | 62.398 | |||
| k2 | 0.0001 | 0.0002 | 0.0002 | 29.6852 | |||
| 100 | R2 | 0.987 | 0.606 | 0.987 | 0.887 | ||
| RMSE | 9.734 | 19.190 | 24.534 | 12.479 | |||
| qe | 85.389 | 473.750 | 95.008 | 0.001 | |||
| k2 | 0.001 | 64.717 | 0.003 | 110.570 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu’azu, N.D. Insight into ANN and RSM Models’ Predictive Performance for Mechanistic Aspects of Cr(VI) Uptake by Layered Double Hydroxide Nanocomposites from Water. Water 2022, 14, 1644. https://0-doi-org.brum.beds.ac.uk/10.3390/w14101644
Mu’azu ND. Insight into ANN and RSM Models’ Predictive Performance for Mechanistic Aspects of Cr(VI) Uptake by Layered Double Hydroxide Nanocomposites from Water. Water. 2022; 14(10):1644. https://0-doi-org.brum.beds.ac.uk/10.3390/w14101644
Chicago/Turabian StyleMu’azu, Nuhu Dalhat. 2022. "Insight into ANN and RSM Models’ Predictive Performance for Mechanistic Aspects of Cr(VI) Uptake by Layered Double Hydroxide Nanocomposites from Water" Water 14, no. 10: 1644. https://0-doi-org.brum.beds.ac.uk/10.3390/w14101644