Chiroptical Properties of Amino Acids: A Density Functional Theory Study
Abstract
:1. Introduction
2. Procedures of Quantum Computations
- μ and m are the electric and magnetic moments, respectively, « ● » is the dot product
- and Im means the imaginary part of a complex number.
3. Results and Discussion
3.1. Conformational Analysis of l-valine and l-isovaline
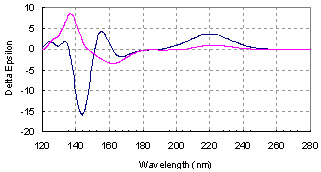
3.2. Computed ECD Spectra of l-valine and l-isovaline
4. Conclusions
Acknowledgements
References
- Jordan, I.K.; Kondrashov, F.A.; Adzhubei, I.A.; Wolf, Y.I.; Koonin, E.V.; Kondrashov, A.S.; Sunyaev, S. A universal trend of amino acid gain and loss in protein evolution. Nature 2005, 433, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J. Amino Acids and the Asymmetry of Life; Springer: Heidelberg, Germany/New York, NY, USA, 2008. [Google Scholar]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Kvenvolden, K; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Muñoz Caro, G.M.; Bredehöft, J.H.; Jessberger, E.K.; Thiemann, W. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 2004, 101, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 2009, 44, 1323–1330. [Google Scholar] [CrossRef]
- Oró, J. Comets and the formation of biochemical compounds on the primitive Earth. Nature 1961, 190, 389–390. [Google Scholar] [CrossRef]
- Cronin, J.R.; Chang, S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In The chemistry of life’s origins; Greenberg, J.M., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Kobayashi, K.; Kasamatsu, T.; Kaneko, T.; Koike, J.; Oshima, T.; Saito, T.; Yamamoto, T.; Yanagawa, H. Formation of amino acid precursors in cometary ice environments by cosmic radiation. Adv. Space Res. 1995, 16, 21–26. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones Segovia, A.; Rosenbauer, H.; Thiemann, W.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef]
- Bossa, J.-B.; Duvernay, F.; Theulé, P.; Borget, F.; d’Hendecourt, L.; Chiavassa, T. Methylammonium methylcarbamate thermal formation in interstellar ice analogs: A glycine salt precursor in protostellar environments. Astron. Astrophys. 2009, 506, 601–608. [Google Scholar] [CrossRef]
- Pizzarello, S. The Chemistry of Life’s Origin: A Carbonaceous Meteorite Perspective. Acc. Chem. Res. 2006, 39, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, W.; Braun, E. Photochemische Erzeugung optisch aktiver Stoffe. Die Naturwissenschaften 1929, 17, 227–228. [Google Scholar] [CrossRef]
- Kuhn, W; Knopf, E. Photochemische Erzeugung optisch aktiver Stoffe. Naturwissenschaften 1930, 18, 183. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Nahon, L.; Alcaraz, C.; Bredehöft, J.H.; Hoffmann, S.V.; Barbier, B.; Brack, A. Asymmetric Vacuum UV photolysis of the Amino Acid Leucine in the Solid State. Angew. Chem. Int. Ed. 2005, 44, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Filippi, J.-J.; Meinert, C.; Hoffmann, S.V.; Bredehöft, J.H.; Nahon, L. Photolysis of rac-Leucine with Circularly Polarized Synchrotron Radiation. Chem. Biodiv. 2010, in press. [Google Scholar] [CrossRef]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular Polarization in Star-Formation Regions: Implications for Biomolecular Homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Thiemann, W.; Rosenbauer, H. Molecular parity violation via comets? Chirality 1999, 11, 575–582. [Google Scholar] [CrossRef]
- Cerf, C.; Jorissen, A. Is amino-acid homochirality due to asymmetric photolysis in space ? Space Sci. Rev. 2000, 92, 603–612. [Google Scholar] [CrossRef]
- Jorissen, A.; Cerf, C. Asymmetric photoreactions as the origin of biomolecular homochirality: a critical review. Orig. Life Evol. Biosph. 2002, 32, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Thiemann, W. Photochemical concepts on the origin of biomolecular asymmetry. Orig. Life Evol. Biosph. 2004, 34, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Takahashi, J-I.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett. 2007, 254, 106–114. [Google Scholar] [CrossRef]
- Thiemann, W.; Rosenbauer, H.; Meierhenrich, U.J. Conception of the ‘chirality-experiment’ on esa's mission rosetta to comet 46P/wirtanen. Adv. Space Res. 2001, 27, 323–328. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; Muñoz Caro, G.M.; Dartois, E.; d’Hendecourt, L.; Deboffle, D.; Auger, G.; Blanot, D.; Bredehöft, J.H.; Nahon, L. The effects of circularly polarized light on amino acid enantiomers produced by the UV irradiation of interstellar ice analogs. Astron. Astrophys. 2006, 457, 741–751. [Google Scholar] [CrossRef]
- Thiemann, W.; Meierhenrich, U.J. ESA Mission ROSETTA Will Probe for Chirality of Cometary Amino Acids. Orig. Life Evol. Biosph. 2001, 31, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Goesmann, F.; Rosenbauer, H.; Roll, R.; Szopa, C.; Raulin, F.; Sternberg, R.; Israel, G.; Meierhenrich, U.J.; Thiemann, W.; Munoz-Caro, G. Cosac, The Cometary Sampling and Composition Experiment on Philae. Space Sci. Rev. 2007, 128, 257–280. [Google Scholar] [CrossRef]
- Gekko, K.; Matsuo, K. Vacuum-Ultraviolet Circular Dichroism Analysis of Biomolecules. Chirality 2006, 18, 329–334. [Google Scholar] [CrossRef]
- Bredehöft, J.H.; Breme, K.; Meierhenrich, U.J.; Hoffmann, S.V.; Thiemann, W. Chiroptical Properties of Diamino Carboxylic Acids. Chirality 2007, 19, 570–573. [Google Scholar] [CrossRef]
- Kaneko, F.; Yagi-Watanabe, K.; Tanaka, M.; Nakagawa, K. Natural Circular Dichroism Spectra of Alanine and Valine Films in Vaccum Ultraviolet Region. J. Phys. Soc. Jpn. 2009, 78, 013001:1–013001:4. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J.V.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M.W.; Johnson, B.G.; Chen, W.; Wong, M.W.; Andres, J.L.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Bour, P. Simulation of Electronic Circular Dichroism with Rigid Kohn-Sham Orbitals: A Computational Experiment. J. Phys. Chem. A 1999, 103, 5099–5104. [Google Scholar] [CrossRef]
- Pecul, M.; Ruud, K.; Helgaker, T. Density functional theory calculation of electronic circular dichroism using London orbitals. Chem. Phys. Lett. 2004, 38, 110–119. [Google Scholar] [CrossRef]
- Stephens, P.J.; McCann, D.M.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J. Determination of the Absolute Configuration of [32](1,4)Barrelenophanedicarbonitrile Using Concerted Time-Dependent Density Functional Theory Calculations of Optical Rotation and Electronic Circular Dichroism. J. Am. Chem. Soc. 2004, 126, 7514–7521. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, E.; Tanaka, K; Verotta, L.; Nakanishi, K.; Berova, N.; Rosini, C. Determination of the absolute configurations of flexible molecules: Synthesis and theoretical simulation of electronic circular dichroism/optical rotation of some pyrrolo[2,3-b]indoline alkaloids - A case study. Chirality 2007, 19, 434–445. [Google Scholar] [PubMed]
- GaussView4; Gaussian, Inc.: Pittsburgh, PA, USA, 2006.
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular dichroism - Principles and Applications, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000; pp. 1–36. [Google Scholar]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Harada, N. ECD Cotton Effect Approximated by the Gaussian Curve and Other Methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]







| Conformers of l-valine | a (°) | b (°) | c (°) | G (H) | ΔG (kJ/mol) | Ni (%) |
|---|---|---|---|---|---|---|
| CC1-CO1-CN1 | - 65.09 | 107.81 | 161.20 | - 402.259301 | 0 | 57.11 |
| CC3-CO5-CN1 | 176.19 | 81.36 | 169.74 | - 402.258267 | 2.72 | 19.09 |
| CC3-CO3-CN3 | 63.77 | 93.75 | 164.41 | - 402.257400 | 4.99 | 7.62 |
| CC1-CO3-CN1 | - 64.83 | - 61.41 | 169.05 | - 402.257202 | 5.51 | 6.18 |
| CC1-CO5-CN3 | - 61.83 | 100.36 | - 78.17 | - 402.256512 | 7.32 | 2.97 |
| CC3-CO5-CN3 | - 177.86 | 74.45 | - 63.29 | - 402.255997 | 8.68 | 1.72 |
| CC2-CO5-CN3 | 65.86 | 90.48 | - 78.67 | - 402.255919 | 8.88 | 1.59 |
| CC2-CO3-CN1 | 66.46 | - 80.25 | 170.29 | - 402.255659 | 9.56 | 1.20 |
| CC2-CO5-CN1 | - 177.29 | - 103.76 | - 55.53 | - 402.255367 | 10.33 | 0.88 |
| CC1-CO3-CN3 | - 62.34 | - 73.94 | - 65.18 | - 402.254890 | 11.58 | 0.53 |
| CC1-CO4-CN2 | - 59.19 | - 27.97 | 71.21 | - 402.254699 | 12.08 | 0.44 |
| CC2-CO3-CN3 | 66.45 | - 83.21 | - 67.26 | - 402.254449 | 12.74 | 0.33 |
| CC2-CO4-CN2 | 71.40 | - 19.53 | 64.27 | - 402.254102 | 13.65 | 0.23 |
| CC3-CO4-CN2 | - 165.38 | 1.57 | 68.87 | - 402.253352 | 15.62 | 0.10 |
| Conformers of l-isovaline | a′ (°) | b′ (°) | c′ (°) | G (H) | ΔG (kJ/mol) | Ni (%) |
|---|---|---|---|---|---|---|
| CC3-CO5-CN1 | - 58.65 | 116.70 | 173.69 | - 402.260832 | 0 | 45.75 |
| CC2-CO5-CN1 | - 178.95 | 109.38 | 175.79 | - 402.259992 | 2.21 | 18.79 |
| CC3-CO6-CN1 | - 60.04 | - 35.03 | 178.53 | - 402.259440 | 3.66 | 10.47 |
| CC1-CO3-CN1 | 63.79 | 87.52 | 176.02 | - 402.259037 | 4.71 | 6.83 |
| CC1-CO1-CN1 | 58.37 | - 128.60 | 177.65 | - 402.258809 | 5.31 | 5.36 |
| CC3-CO7-CN3 | - 51.81 | 139.77 | 83.15 | - 402.258525 | 6.06 | 3.97 |
| CC3-CO3-CN2 | - 58.36 | 100.69 | - 78.12 | - 402.258061 | 7.28 | 2.43 |
| CC1-CO1-CN2 | 63.11 | - 104.99 | - 55.65 | - 402.257413 | 8.98 | 1.22 |
| CC2-CO4-CN3 | - 56.49 | - 26.85 | 66.56 | - 402.257375 | 9.08 | 1.17 |
| CC3-CO1-CN2 | - 58.52 | - 77.94 | - 63.91 | - 402.256869 | 10.41 | 0.69 |
| CC2-CO2-CN3 | - 170.84 | 136.57 | 91.62 | - 402.256855 | 10.44 | 0.68 |
| CC2-CO3-CN2 | - 176.70 | 93.45 | - 68.44 | - 402.256749 | 10.72 | 0.60 |
| CC2-CO6-CN1 | - 178.54 | - 58.33 | - 179.59 | - 402.256739 | 10.75 | 0.60 |
| CC1-CO3-CN2 | 62.35 | 80.52 | - 68.14 | - 402.256619 | 11.06 | 0.53 |
| CC2-CO1-CN2 | - 175.29 | - 103.38 | - 53.81 | - 402.256494 | 11.39 | 0.46 |
| CC2-CO4-CN3 | - 172.29 | - 17.68 | 70.12 | - 402.255819 | 13.16 | 0.23 |
| CC1-CO4-CN3 | 70.08 | - 4.62 | 61.99 | - 402,255366 | 14.35 | 0.14 |
| CC1-CO2-CN3 | 65.73 | - 169.89 | 60.42 | - 402,255011 | 15.28 | 0.10 |
© 2010 by the authors; licensee MDPI, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adrian-Scotto, M.; Antonczak, S.; Bredehöft, J.H.; Hoffmann, S.V.; Meierhenrich, U.J. Chiroptical Properties of Amino Acids: A Density Functional Theory Study. Symmetry 2010, 2, 935-949. https://0-doi-org.brum.beds.ac.uk/10.3390/sym2020934
Adrian-Scotto M, Antonczak S, Bredehöft JH, Hoffmann SV, Meierhenrich UJ. Chiroptical Properties of Amino Acids: A Density Functional Theory Study. Symmetry. 2010; 2(2):935-949. https://0-doi-org.brum.beds.ac.uk/10.3390/sym2020934
Chicago/Turabian StyleAdrian-Scotto, Martine, Serge Antonczak, Jan Hendrik Bredehöft, Søren V. Hoffmann, and Uwe J. Meierhenrich. 2010. "Chiroptical Properties of Amino Acids: A Density Functional Theory Study" Symmetry 2, no. 2: 935-949. https://0-doi-org.brum.beds.ac.uk/10.3390/sym2020934