Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects
Abstract
:1. Introduction
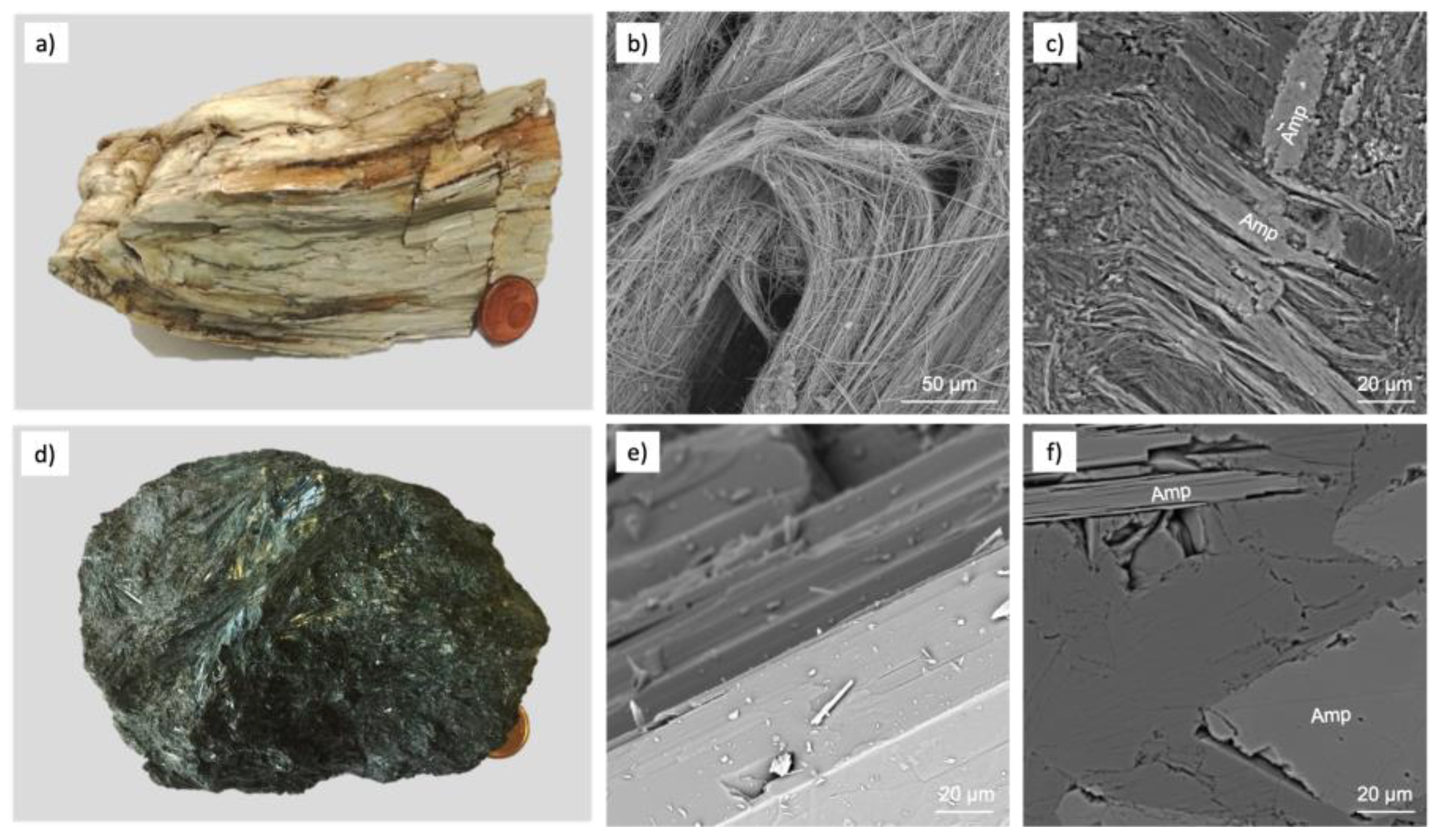
2. Mineralogical vs. Commercial Definitions
- Cleavage fragments: proposed by the Occupational Safety and Health Administration (OSHA) in 1992. It is a term that refers to amphibole (crocidolite, amosite, anthophyllite, tremolite, actinolite) or serpentine (antigorite and lizardite) that, from a morphological point of view, cleave into fragments rather than separate longitudinally into fibrils like asbestos varieties. Therefore, they have the same chemical composition of asbestos species, but in geometrical ratios, they fall within the definition of fibre, although they are not asbestiform. Considering the indexing of crystal faces, monoclinic amphiboles have perfect cleavage along the 110 face, and orthorhombic amphiboles have perfect cleavage along the 210 face [10].
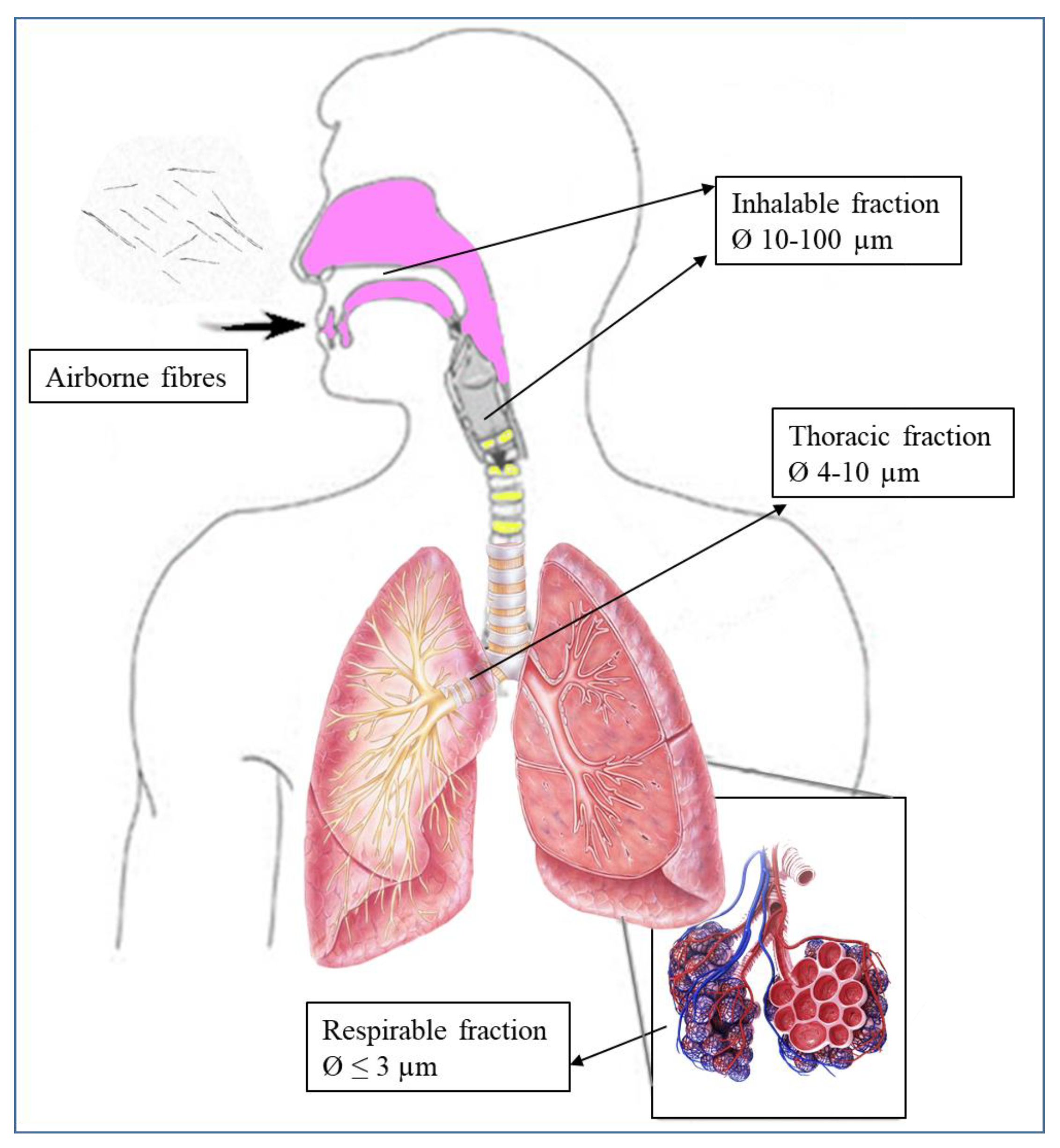
- Elongate mineral particles (EMP): proposed by the National Institute for Occupational Safety and Health (NIOSH) [11] to describe all particles sharing specific attributes, though applicable to a broad class of particle types. In particular, the term refers to mineral particles with a length ≥ 5 μm and a minimum aspect ratio of 3:1, which correspond to breathable size [12], avoiding using the term “fibre”, which leads to misunderstandings in the definition.
3. Studies on Asbestos/Nonasbestos-Related Diseases and Exposure
Potential Health Effects of Elongated Mineral Particles
4. Regulatory Framework
- The NIOSH and the USA Environmental Protection Agency (EPA) [71] propose a very cautious approach favouring exposed workers. Therefore, quantification methods of asbestos (Method 7400 ‘A’ and ‘B’) are much more stringent [72,73]. In fact, to some scientific community members, any very thin and long mineral with a high aspect ratio can be called fibrous ([74] and references within). NIOSH, OSHA, the WHO and the EPA favour including cleavage fragments within fibre counts, taking into account length and diameter.
- The American Society for Testing and Materials (ASTM) restricts the counting field only to those mineral phases whose appearance meets the specific characteristics (curvature, flaking, presence of fibrils at the apex of the beam) described by NIOSH and length > 10 μm or width < 1 μm [75].
Counting Criteria and Cleavage Fragments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Monograph on the Evaluation of the Risk to Human-Asbestos—Supplement 7; IARC Scientific Publication International Agency for Research on Cancer: Lyon, France, 1987. [Google Scholar]
- Pisu, R.; Cinus, S.; Demuru, S.; Di Gregorio, A.; Manca, P.; Marras, M.; Perezzani, S. Direttive Generali per La Redazione del Piano Regionale di Protezione, Decontaminazione, Smaltimento e Bonifica Dell’Ambiente ai Fini Della Difesa dai Pericoli Derivanti Dall’Amianto; Regione Autonoma della Sardegna Assessorato della difesa dell’Ambiente: Cagliari, Italy, 2008. [Google Scholar]
- Van Orden, D.R.; Allison, K.A.; Lee, R.J. Differentiating amphibole asbestos from non-asbestos in a complex mineral environment. Indoor Built Environ. 2008, 17, 58–68. [Google Scholar] [CrossRef]
- Boulanger, G.; Andujar, P.; Pairon, J.C.; Billon-Galland, M.A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibres to assess asbestos exposure: A review of fibre size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO (World Health Organization). The World Health Report 1997—Conquering Suffering, Enriching Humanity; WHO: Geneva, Switzerland, 1997. [Google Scholar]
- Militello, G.M.; Sanguineti, E.; Yus González, A.; Gaggero, L. Asbestos amphiboles: Effects of comminution on tremolite and actinolite regulated and unregulated fibres. Episodes 2020, 43. [Google Scholar] [CrossRef]
- Ilgren, E.B.; Penna, B.M. The Biology of Cleavage Fragments: A Brief Synthesis and Analysis of Current Knowledge. Indoor Built Environ. 2004, 19, 14. [Google Scholar] [CrossRef]
- National Research Council (US). Asbestiform Fibres Nonoccupational Health Risks; National Academies Press (US): Washington, DC, USA, 1984. [Google Scholar]
- Langer, A.M.; Nolan, R.P.; Addison, J. Distinguishing between amphibole asbestos fibres and elongate cleavage fragment of their non-asbestos analogues. In Mechanism in Fibre Carcinogenesis; Plenum Press: New York, NY, USA, 1991; pp. 253–267. [Google Scholar]
- Shelley, D. Manual of Optical Mineralogy; Elsevier Scientific Publishing Company: New York, NY, USA, 1975; pp. 158–170. [Google Scholar]
- National Institute for Occupational Safety and Health. Current Intelligence Bulletin. 2010. Asbestos Fibres and Other Elongate Mineral Particles: State of the Science and Roadmap for Research. Available online: http://www.cdc.gov/niosh/review/public/099-C/pdfs/AsbestosRoadmapPublicCommentDraftV4.pdf (accessed on 9 August 2010).
- Williams, C.; Dell, L.; Adams, R.; Rose, T.; Van Orden, D. State-of-the-science assessment of non-asbestos amphibole exposure: Is there a cancer risk? Environ. Geochem. Health 2013, 35, 357–377. [Google Scholar] [CrossRef]
- Keane, M.J.; Stephens, J.W.; Zhong, B.Z.; Miller, W.E.; Wallace, W.E. A study of the effect of chrysotile fibre surface composition on genotoxicity in vitro. J. Toxicol. Environ. Health Part A 1999, 57, 529–541. [Google Scholar] [CrossRef]
- Cook, W.E. Pulmonary asbestosis. Br. Med. J. 1927, 2, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Doll, R. Mortality for lung cancer in asbestos workers. Br. J. Ind. Med. 1955, 12, 81–86. [Google Scholar] [CrossRef] [Green Version]
- IARC. Monograph in Evaluation of the Carcinogenic Risk of Chemical to Man. In Asbestos; World Health Organization (WHO): Lyon, France, 1977; p. 106. [Google Scholar]
- World Health Organisation. WHO Air Quality Guidelines. In Asbestos, 2nd ed; Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Luberto, F.; Amendola, P.; Belli, S.; Bruno, C.; Candela, S.; Grignoli, M.; Comba, P. Mortality study of asbestos cement workers in Emilia-Romagna. Epidemiol. Prev. 2004, 28, 239–246. [Google Scholar] [PubMed]
- Szeszenia-Dąbrowska, N.; Wilczyńska, U. Medical monitoring of asbestos-exposed workers: Experience from Poland Beata Świątkowska. Bull. World Health Organ. 2016, 94, 599–604. [Google Scholar]
- European Commission. Directive 2003/18/EC and Directive/2009/148/EC, Protection of Workers from the Risks Related to Exposure to Asbestos at Work; European Commission: Brussels, Belgium; Luxembourg, 2009. [Google Scholar]
- Gaggero, L.; Sanguineti, E.; Yus González, A.; Militello, G.M.; Scuderi, A.; Parisi, G. Airborne asbestos fibres monitoring in tunnel excavation. J. Environ. Manag. 2017, 196, 583–593. [Google Scholar] [CrossRef]
- Ishida, T.; Fujihara, N.; Nishimura, T.; Funabashi, H.; Hirota, R.; Ikeda, T.; Kuroda, A. Live-cell imaging of macrophage phagocytosis of asbestos fibres under fluorescence microscopy. Genes Environ. 2019, 41, 14. [Google Scholar] [CrossRef] [Green Version]
- IARC. Biological Agents; IARC Monographs on the Evaluation Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100B. [Google Scholar]
- La Maestra, S.; Micale, R.T.; Ferretti, M.; Izzotti, A.; Gaggero, L. Attenuation of oxidative stress and chromosomal aberrations in cultured macrophages and pulmonary cells following self-sustained high temperature synthesis of asbestos. Sci. Rep. 2020, 10, 8581. [Google Scholar] [CrossRef] [PubMed]
- Solbes, E.; Harper, R.W. Biological responses to asbestos inhalation and pathogenesis of asbestos-related benign and malignant disease. J. Investig. Med. 2018, 66, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ngamwong, Y.; Tangamornsuksan, W.; Lohitnavy, O.; Chaiyakunapruk, N.; Scholfield, C.N.; Reisfeld, B. Additive Synergism between Asbestos and Smoking in Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135798. [Google Scholar] [CrossRef]
- Harper, M. 10th Anniversary Critical Review: Naturally occurring asbestos. J. Environ. Monit. 2008, 10, 1394–1408. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.F.; Gibbs, G.W. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul. Toxicol. Pharmacol. 2008, 52, S154–S186. [Google Scholar] [CrossRef] [PubMed]
- Pott, F.; Roller, M.; Ziem, U.; Reiffer, F.J.; Bellmann, B.; Rosenbruch, M.; Huth, F. Carcinogenicity studies on natural and man-made fibres with the intraperitoneal test in rats. In Non-Occupational Exposure to Mineral Fibres; Bignon, J., Peto, J., Saracci, R., Eds.; International Agency for Research on Cancer: Lyon, France, 1989; pp. 173–179. [Google Scholar]
- Hamra, G.B.; Loomis, D.; Dement, J. Examining the association of lung cancer and highly correlated fibre size-specific asbestos exposures with a hierarchical Bayesian model. Occup. Environ. Med. 2014, 71. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Berry, G.; Skidmore, J.W.; Timbrell, V. The effects of the inhalation of asbestos in rats. Br. J. Cancer 1974, 29, 252–269. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Yuen, R.; Ashley, R. Short, thin asbestos fibres contribute to the development of human malignant mesothelioma: Pathological evidence. Intern. J. Hyg. Environ. Health 2005, 208, 201–210. [Google Scholar] [CrossRef]
- Davis, J.M.G.; Addison, J.; McIntosh, C.; Miller, B.G.; Niven, K. Variations in the carcinogenicity of tremolite dust samples of differing morphology. Ann. N.Y. Acad. Sci. 1991, 643, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Adib, G.; Labreche, F.; De, G.L.; Dion, C.; Dufresne, A. Short, fine and WHO asbestos fibres in the lungs of Quebec workers with an asbestos-related disease. Am. J. Ind. Med. 2013, 56, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Brown, G.M.; Brown, D.M.; Bolton, R.E.; Davis, J.M.G. Inflammation generating potential of long and short fibre amosite asbestos samples. Br. J. Ind. Med. 1989, 46, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, K.; Szymaniec, S.; Li, X.Y.; Brown, D.M.; Brown, G.M. Inflammation and immunomodulation caused by short and long amosite asbestos samples. In Mecanisms in Fibre Carcinogenesis; Brown, R.C., Hoskins, J., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 121–130. [Google Scholar]
- Berman, W.; Crump, K.; Chatfield, E.; Davis, J.; Jones, A. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995, 15, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Goodglick, L.A.; Kane, A.B. Cytotoxicity of long and short crocidolite asbestos fibres in vitro and in vivo. Cancer Res. 1990, 50, 5153–5163. [Google Scholar] [PubMed]
- Davis, J.M.G.; Addison, J.; Bolton, R.E. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 1986, 67, 415–430. [Google Scholar]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particle dimension to carcinogenicity in amphibole asbestos and other fibrous minerals. J. Natl. Cancer Inst. 1981, 67, 965–976. [Google Scholar] [PubMed]
- Brown, G.M.; Cowie, H.; Davis, J.M.G.; Donaldson, K. In vitro assays for detecting carcinogenic mineral fibres: A comparison of two assays and the role of fibre size. Carcinogenesis 1986, 7, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.M.; Beswick, P.H. Differential release of superoxide anions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup. Environ. Med. 1995, 52, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, M. Effects of fibre characteristics on lung deposition, retention, and disease. Environ. Health Perspect. 1990, 88, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dement, J.M.; Kuempel, E.D.; Zumwalde, R.D.; Smith, R.J.; Stayner, L.T.; Loomis, D. Development of a fibre size-specific job-exposure matrix for airborne asbestos fibres. Occup. Environ. Med. 2008, 65, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.W.; Crump, K.S. A meta-analysis of asbestos-related cancer risk that addresses fibre size and mineral type. Crit. Rev. Toxicol. 2008, 38 (Suppl. 1), 49–73. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.M.; Moodie, C.A.; Lawrence, G.A.; Ponterfract, R.D. Chronic effects of ingested asbestos in rats. Arch. Environ. Contam. Toxicol. 1977, 6, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Patel-Mandlik, K.J.; Millette, J.R. Chrysotile asbestos in kidney cortex of chronically gavaged rats. Arch. Environ. Contam. Toxicol. 1983, 12, 247–255. [Google Scholar] [CrossRef]
- Weinzweig, M.; Richards, R.J. Quantitative assessment of chrysotile fibrils in the bloodstream of rats which have ingested the mineral under dietary conditions. Environ. Res. 1983, 31, 245–255. [Google Scholar] [CrossRef]
- Dodson, R.; Atkinson, M.A.L.; Levin, J.L. Asbestos fibre length as related to potential pathogenicity: A critical review. Am. J. Ind. Med. 2003, 44, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Zoboli, A.; Vigliaturo, R.; Gieré, R.; Bonasoni, M.P.; Sala, O.; Gualtieri, A.F. Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study. Minerals 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Timbrell, V.; Griffiths, D.; Pooley, F. Possible importance of fiber diameters of South African Amphiboles. Nature 1971, 232, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.; Mossman, B. Mineralogical features associated with cytotoxic and proliferative effects of fibrous talc and asbestos on tracheal epithelial and pleural mesothelial cells. J. Toxic Appl. Pharmacol. 1997, 147, 153–160. [Google Scholar] [CrossRef]
- Pott, F.; Huth, F.; Friedrichs, K.H. Tumorigenic effects of fibrous dusts in experimental animals. Environ. Health Perspect. 1974, 9, 313–315. [Google Scholar]
- Addison, L.; McConnel, E.E. A review of carcinogenicity studies of asbestos and non-asbestos tremolite and other amphiboles. Regul. Toxicol. Pharmacol. 2008, 52, S187–S199. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.G.; Bailey, K.F.; Kelse, J.W.; Lee, R.J. The importance of width in asbestos fibre carcinogenicity and its implications for public policy. Am. Ind. Hyg. Assoc. J. 1993, 54, 239. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Aldieri, E.; Bergandi, L.; Tomatis, M.; Fenoglio, I.; Costamagna, C.; Fubini, B.; Bosia, A.; Ghigo, D. Long and short fibre amosite asbestos alters at a different extent the redox metabolism in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2003, 193, 106–115. [Google Scholar] [CrossRef]
- Wagner, J.C.; Chamberlain, M.; Brown, R.C.; Berry, G.; Pooley, F.D.; Davies, R.; Griffiths, D.M. Biological effects of tremolite. Br. J. Cancer 1982, 45, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Berman, D.W.; Crump, K.S. Technical Support Document for a Protocol to Assess Asbestos Related Risk; U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- McConnell, E.E.; Axten, C.; Hesterberg, T.W.; Chevalier, J.; Miiller, W.C.; Everitt, J.; Oberdorster, G.; Chase, G.R.; Thevenaz, P.; Kotin, P. Studies on the inhalation toxicology of two fibreglasses and amosite asbestos in the Syrian golden hamster. Part II. Results of chronic exposure. Inhal. Toxicol. 1999, 11, 785–835. [Google Scholar] [CrossRef] [PubMed]
- Vallero, D.A.; Beard, M.E. Selecting Appropriate Analytical Methods to Characterize Asbestos in Various Media. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2009, 13, 249–260. [Google Scholar] [CrossRef]
- Mossman, B. Assessment of the pathogenic potential of asbestiform vs. non asbestiform particulates (cleavage fragments) in in vitro (cell or organ culture) models and bioassays. Regul. Toxicol. Pharmacol. 2008, 52, S200–S203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossman, B.T.; Lippmann, M.; Hesterberg, T.W.; Kelsey, K.T.; Barchowsky, A.; Bonner, J.C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health Part B Crit. Rev. 2011, 14, 76–121. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, E. A Procedure for Quantitative Description of Fibrosity in Amphibole Minerals. In Proceedings of the ASTM Michael E. Beard Asbestos Conference, San Antonio, TX, USA, 28–29 January 2012. [Google Scholar]
- Belardi, G.; Vignaroli, G.; Trapasso, F.; Pacella, A.; Passeri, D. Detecting asbestos fibres and cleavage fragments produced after mechanical tests on ophiolite rocks: Clues for the asbestos hazard evaluation. J. Mediterr. Earth Sci. 2018, 10, 63–78. [Google Scholar] [CrossRef]
- Stayner, L.; Kuempel, E.; Gilbert, S.; Hein, M.; Dement, J. An epidemiological study of the role of chrysotile asbestos fibre dimensions in determining respiratory disease risk in exposed workers. Occup. Environ. Med. 2008, 65, 613–619. [Google Scholar] [CrossRef]
- Loomis, D.; Dement, J.M.; Wolf, S.H.; Richardson, D.B. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup. Environ. Med. 2009, 66, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Dement, J.; Richardson, D.; Wolf, S. Asbestos fibre dimensions and lung cancer mortality among workers exposed to chrysotile. Occup. Environ. Med. 2010, 67, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomis, D.; Dement, J.M.; Elliott, L.; Richardson, D.; Kuempel, E.D.; Stayner, L. Increased lung cancer mortality among chrysotile asbestos textile workers is more strongly associated with exposure to long thin fibres. Occup. Environ. Med. 2012, 69, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Cavariani, F.; Marconi, A.; Sala, O. Asbestos: Sampling, analytical techniques and limit values. Arch. Issues 2010, 1, 18–28. [Google Scholar]
- Militello, G.M.; Sanguineti, E.; Yus González, A.; Mantovani, F.; Gaggero, L. The Concentration of Asbestos Fibres in Bulk Samples and Its Variation with Grain Size. Minerals 2019, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Perkins, R.L.; Harvey, B.W. Method for the Determination of Asbestos in Bulk Building Materials; U.S. Environmental Protection Agency EPA/600/R-93/116; EPA (Environmental Protection Agency), Office of Research and Development: Washington, DC, USA, 1993. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). Method 7400, Asbestos and other fibres by PCM. In NIOSH Manual of Analytical Methods, 4th ed.; DHSS (NIOSH) Pub. No. 2003-154; NIOSH: Cincinnati, OH, USA, 2003. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). Method 7402: Asbestos by TEM. In NIOSH Manual of Analytical Methods (NMAM), 4th ed.; NIOSH: Cincinnati, OH, USA, 2003. [Google Scholar]
- Gualtieri, A.F. Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; Volume 18, p. 536. ISBN 9780903056656. [Google Scholar]
- Harper, M.; Lee, E.G.; Slaven, J.; Bartley, D. An inter-laboratory study to determine the effectiveness of procedures for discriminating amphibole asbestos fibres from amphibole cleavage fragments in fibre counting by phase-contrast microscopy. Ann. Occup. Hyg. 2012, 56, 645–659. [Google Scholar] [CrossRef]
- Agence Nationale de Sécurité Sanitaire (ANSES). Opinion of the French Agency for Food, Environmental and Occupational Health & Safety. In Health Effects and the Identification of Cleavage Fragments of Amphiboles from Quarried Minerals; Request No. 2014_SA_0196; Agence Nationale de Sécurité Sanitaire (ANSES): Paris, France, 2015. [Google Scholar]
- AHERA (Asbestos Hazardous Emergency Response Act). Interim Transmission Electron Microscopy Analytical Methods—Mandatory and Non-Mandatory—And Mandatory Section to Determine Completion of Response. Fed. Regist. 1987, 52, 41857–41897. [Google Scholar]
- Harris, K.E.; Bunker, K.L.; Strohmeier, B.R.; Hoch, R.; Lee, R.J. Discovering the True Morphology of Amphibole Minerals: Complementary TEM and FESEM Characterization of Particles in Mixed Mineral Dust; Modern Research and Educational Topics in Microscopy; Méndez-Vilas, A., Díaz, J., Eds.; RJ Lee Group, Inc.: Monroeville, PA, USA, 2007. [Google Scholar]
- Van Orden, D.R.; Lee, R.J.; Allison, K.A.; Addison, J. Width distributions of asbestos and non-asbestos amphibole minerals. Indoor Built Environ. 2009, 18, 531–540. [Google Scholar] [CrossRef]
- Hwang, J.; Ramachandran, G.; Raynor, P.C.; Alexander, B.H.; Mandel, J.H. The Relationship Between Various Exposure Metrics for Elongate Mineral Particles (EMP) in the Taconite Mining and Processing Industry. J. Occup. Environ. Hyg. 2014, 11, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W. Comments on NIOSH asbestos roadmap—Animal bioassays. In Proceedings of the Workshop on the NIOSH Research Roadmap on Asbestos Fibres and Other Elongated Mineral Particles, Washington, DC, USA, 30 March 2009; National Institute of Occupational Safety and Health: Washington, DC, USA, 2009. [Google Scholar]

| Mineral Type | Study Types | Critical Size of Fibres | References | ||
|---|---|---|---|---|---|
| Length | Width | Aspect Ratio | |||
| Actinolite | In vivo | <0.2 µm | - | 3:1 | [29] |
| Not specified | Mathematical model | >1.5 µm | <0.25 µm | 3:1 | [30] |
| Crocidolite | In vivo | >3 µm | - | 3:1 | [31] |
| Not specified | Observational | ≤5 µm | ≤0.25 µm | 3:1 | [32] |
| Tremolite | In vivo | >5 µm | ≤0.5 µm | 3:1 | [33] |
| Not Specified | Observational | <5 µm | <3 µm | 3:1 | [34] |
| Tremolite and other | In vivo | >5 µm | - | 3:1 | [35,36,37,38,39] |
| EMP Amphiboles | In vitro | >8 µm | <0.25 µm | 3:1 | [40] |
| Tremolite and other | In vitro | >10 µm | - | 3:1 | [6,35,36,41,42] |
| Tremolite | In vivo | >10 µm | <0.5 µm | 3:1 | [39] |
| Not specified | In vivo | >20 µm | <1 µm | 3:1 | [43] |
| EMP | Cohort studies | >40 µm | <3 µm | 3:1 | [44] |
| Asbestos group | Meta-analysis | - | <0.4 µm | 3:1 | [45] |
| Country | Single Particle | Reference |
|---|---|---|
| South Africa | L > 8 µm; D < 0.25 µm | [40] |
| N. A. | A/R ≥ 5:1 | [77] |
| N. A. | A/R 20:1–100:1 | [71] |
| N. A. | L > 10 µm; D < 0.4 µm; A/R > 25:1 | [58] |
| Libby | D < 0.5 µm; A/R 20:1–100:1 | [78] |
| Brazil, Colorado, Labrador | D < 1.5 µm; A/R > 20:1 | [63] |
| North Carolina, New York, Virginia | A/R > 5:1 | [3] |
| Jamestown, North Carolina, South Africa, Italy, Libby, Austria, Gouverneur, West Greenland, Homestake, Shinness | D < 0.5 µm; A/R > 16:1 | [79] |
| N.A. | D < 1 µm | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militello, G.M.; Gaggero, L.; La Maestra, S. Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects. Minerals 2021, 11, 525. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050525
Militello GM, Gaggero L, La Maestra S. Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects. Minerals. 2021; 11(5):525. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050525
Chicago/Turabian StyleMilitello, Gaia M., Laura Gaggero, and Sebastiano La Maestra. 2021. "Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects" Minerals 11, no. 5: 525. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050525






