Lead Recovery from Solid Residues of Copper Industry Using Triethylenetetramine Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppelaar, R.H.E.M.; Koppelaar, H. The Ore Grade and Depth Influence on Copper Energy Inputs. Biophys. Econ. Resour. Qual. 2016, 11, 2–16. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.G.; Muir, D.M. Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 2007, 86, 191–205. [Google Scholar] [CrossRef]
- Lundstrom, M.; Lippo, J.; Karonen, J.; Aromaa, J. Dissolution of six sulfide concentrates in the hydrocopper® environment. In Proceedings of the Southern African Institute of Mining and Metallurgy Base Metals Conference, Kasane, Botswana, 27–31 July 2009; pp. 127–138. [Google Scholar]
- Fleming, C.A. Basic iron sulphate—A potential killer for pressure oxidation processing of refractory gold concentrates if not handled appropriately. Miner. Metall. Proc. 2010, 27, 81–88. [Google Scholar]
- Long, H. A Fundamental Study of the Acidic Pressure Oxidation of Orpimeni and Pyrite at High Temperature. Ph.D. Thesis, The University of British Columbia, Vancouver, WA, Canada, 2000. [Google Scholar]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, S.C. Recovery of lead from zinc plant waste. Trans. Indian Inst. Met. 1986, 39, 604–608. [Google Scholar]
- Vinals, J.; Nunez, C.; Carassco, J. Leaching of gold, silver and lead from plumbojarosite-containing hematite tailings in HCl-CaCl2 media. Hydrometallurgy 1991, 26, 179–199. [Google Scholar] [CrossRef]
- Kunda, W.; Veltman, H. Decomposition of jarosite. Metall. Trans. 1979, 10, 439–446. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A.; Patino, F.; Salinas, E.; Rivera, I. Cyanidation kinetics of argentian jarosite in alkaline media. Hydrometallurgy 2000, 55, 153–163. [Google Scholar] [CrossRef]
- Patino, F.; Arenas, A.; Rivera, I.; Cordoba, D.A.; Hernandez, L.; Salinas, E. Decomposition of argentiferous plumbojarosite in CaO media. Rev. Soc. Quim. Mexico 1998, 42, 122–128. [Google Scholar]
- Liu, C.; Ju, S.H.; Zhang, L.B.; Srinivasakannan, C.; Peng, J.H.; Le, T.Q.X.; Guo, Z.Y. Recovery of valuable metals from jarosite by sulphuric acid roasting using microwave and water leaching. Can. Metall. Q. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Ju, S.H.; Zhang, Y.F.; Zhang, Y.; Wang, Y.F. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Roca, A.; Cruells, M.; Patiño, F.; Córdoba, D.A. Characterization and alkaline decomposition–cyanidation kinetics of industrial ammonium jarosite in NaOH media. Hydrometallurgy 2001, 60, 237–246. [Google Scholar] [CrossRef]
- Deng, X.; Liu, W.; Zhang, D.; Chen, L.; Liu, Z.; Yang, T. Hydrothermal desulfurization of spent lead paste based on comproportionation reaction. Sep. Purif. Technol. 2011, 259, 118115. [Google Scholar] [CrossRef]
- Dai, F.; Huang, H.; Chen, B.; Zhang, P.; Hea, Y.; Guo, Z. Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Sep. Purif. Technol. 2019, 224, 237–246. [Google Scholar] [CrossRef]
- Tang, L.; Tang, C.B.; Xiao, J.; Zeng, P.; Tang, M.; Wang, Z.; Zhang, Z. A cleaner process for lead recovery from lead-containing hazardous solid waste and zinc leaching residue via reducing-matting smelting. J. Clean. Prod. 2019, 241, 118328. [Google Scholar] [CrossRef]
- Lyakov, N.K.; Atanasova, D.A.; Vassilev, V.S.; Haralampiev, G.A. Desulphurization of damped battery paste by sodium carbonateand sodium hydroxide. J. Power Sources 2007, 171, 960–965. [Google Scholar] [CrossRef]
- Ning, P.; Pan, J.-Q.; Li, X.; Zhou, Y.; Chen, J.-F.; Wang, J.-X. Accelerated desulphurization of waste lead battery paste in a high-gravity rotating packed bed. Chem. Eng. Process. 2016, 104, 148–153. [Google Scholar] [CrossRef]
- Ma, C.; Shu, Y.; Chen, H. Preparation of high-purity lead oxide from spent lead paste by low temperature burning and hydrometallurgical processing with ammonium acetate solution. RSC Adv. 2016, 6, 21148. [Google Scholar] [CrossRef]
- Sonmeza, M.S.; Kumar, R.V. Leaching of waste battery paste components. Part 2: Leaching and desulphurisationof PbSO4 by citric acid and sodium citrate solution. Hydrometallurgy 2009, 95, 82–86. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solidwastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
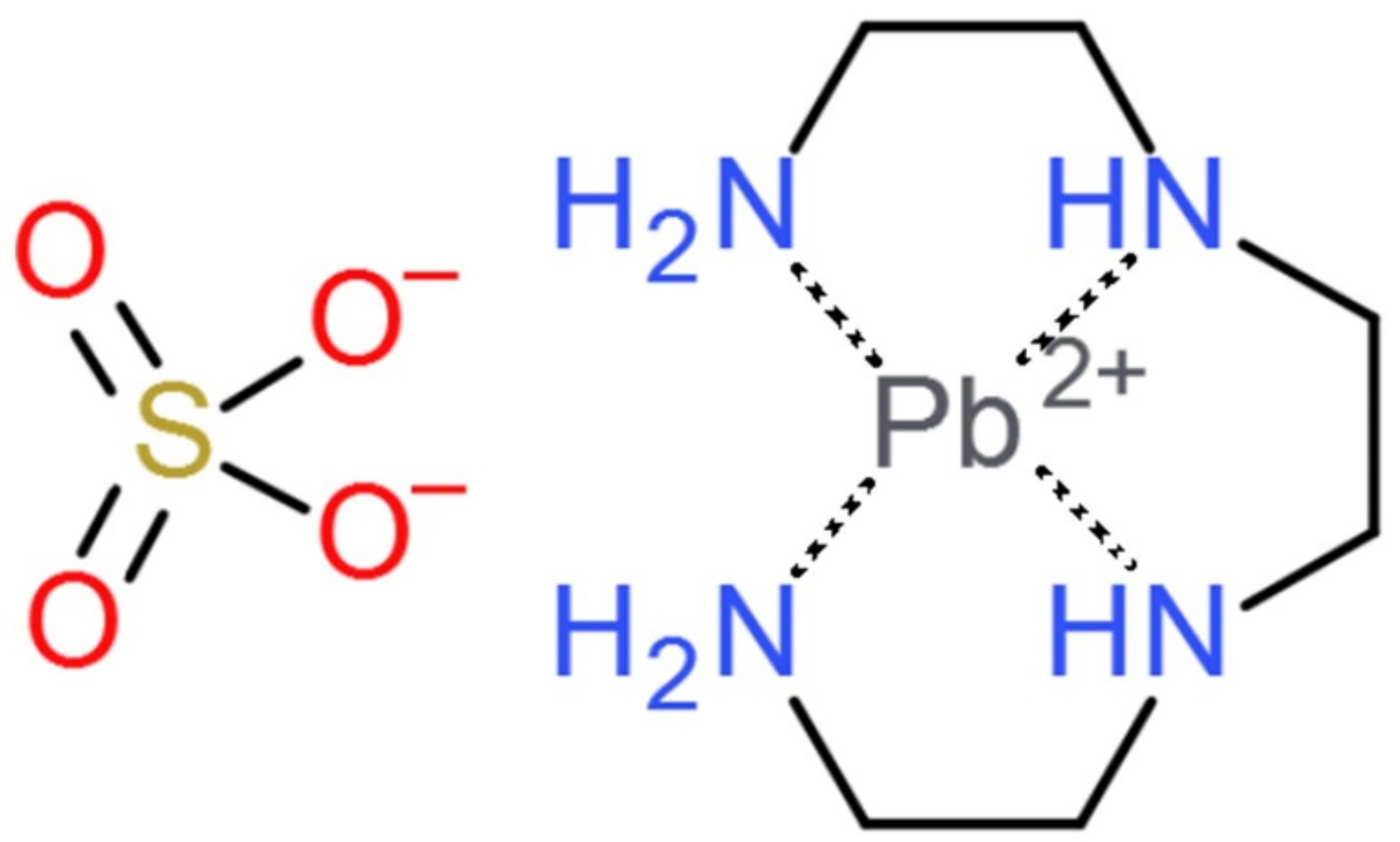
- Chmielarz, A.; Szołomicki, Z.; Kurowski, R.; Mrozowski, J. Triethylenetetramine (TETA)—A powerful leachant in lead recovery. In Proceedings of the 7th International Symposium Hydrometallurgy, Victoria, BC, Canada, 22–25 June 2014. [Google Scholar]
- Wang, L.; Chen, Q.; Jamro, I.A.; Lia, R.D.; Baloch, H.A. Accelerated co-precipitation of lead, zinc and copper by carbon dioxide bubbling in alkaline municipal solid waste incinerator (MSWI) fly ash wash water. RSC Adv. 2016, 6, 20173–20186. [Google Scholar] [CrossRef]

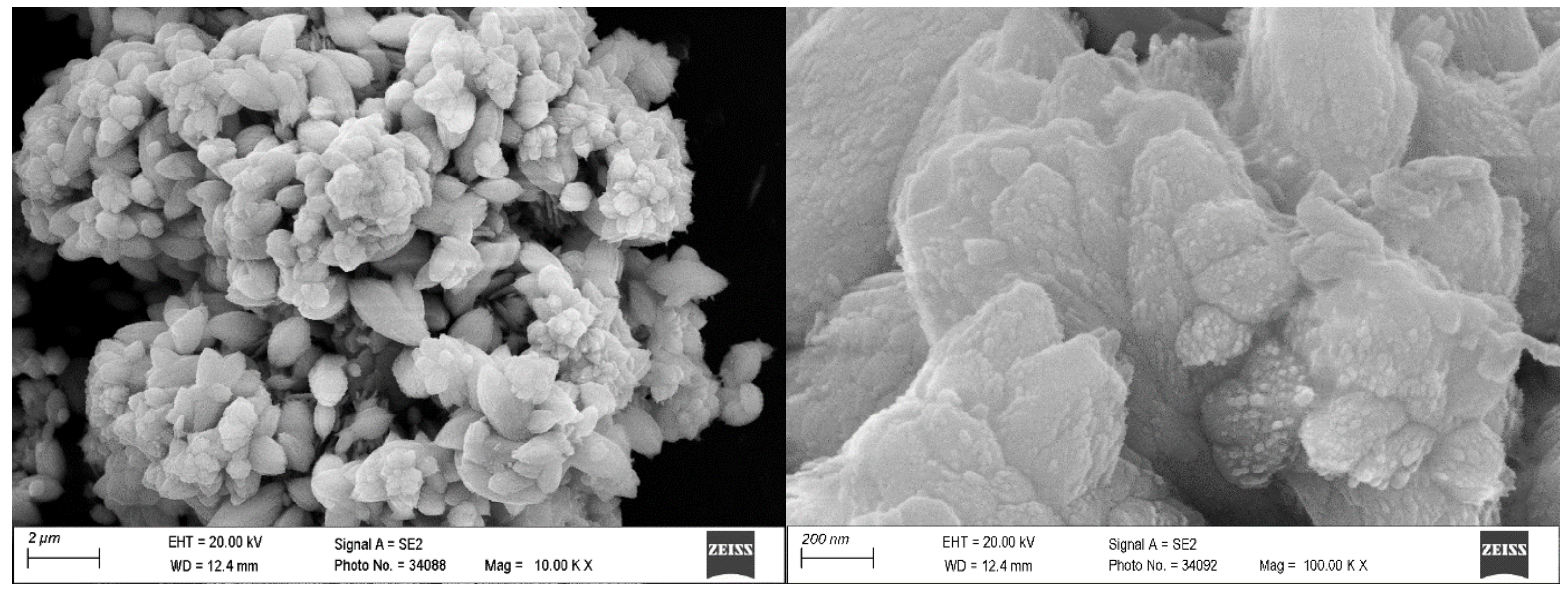
| Sample | Pb | Zn | Cu | Fe | As | Al | Si | Ag | STOT | SSO4 |
|---|---|---|---|---|---|---|---|---|---|---|
| SE | 6.68 | 0.07 | 0.05 | 21.1 | 0.25 | 0.07 | 1.41 | 0.006 | 13.5 | 13.32 |
| PL | 6.37 | 0.05 | 1.25 | 10.4 | 0.54 | 4.72 | 10.95 | 0.077 | 6.75 | 6.61 |
| OR | 19.7 | 0.31 | 0.31 | 29.45 | 0.21 | 0.10 | 0.38 | 0.029 | 11.75 | 10.97 |
| Sample | SE | PL | OR |
|---|---|---|---|
| CPbSOLID/% | 14.8 | 8.9 | 59.2 |
| pHTETA start | 12 | 11.8 | 12 |
| pHTETA final | 11.1 | 11.0 | 11 |
| Mass loss/% | 27.3 | 29.3 | 76.4 |
| Pb leached/% | 91.3 | 37.8 | 86.2 |
| Sample | Pb/% | Fe/% | Cu/% | As/% | Ni/% | Wettness/% |
|---|---|---|---|---|---|---|
| SE | 76 | <0.005 | <0.0025 | <0.01 | 0 | 20 |
| PL | 75.7 | 0.25 | <0.0025 | <0.01 | 0.03 | 24 |
| OR | 75.8 | <0.005 | <0.0025 | <0.01 | 0 | 22 |
| Sample | Pb/% | Fe/% | Cu/% | As/% | Co/% | CaCO3/% | Wett./% |
|---|---|---|---|---|---|---|---|
| SE | <0.0042 | 0.012 | <0.0025 | <0.01 | <0.0025 | 0.8 | 9.4 |
| PL | <0.003 | 0.012 | <0.0025 | <0.01 | <0.0025 | 0.89 | 15 |
| OR | <0.0085 | 0.011 | <0.0025 | <0.01 | <0.0025 | 1 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciszewski, M.; Chmielarz, A.; Szołomicki, Z.; Drzazga, M.; Leszczyńska-Sejda, K. Lead Recovery from Solid Residues of Copper Industry Using Triethylenetetramine Solution. Minerals 2021, 11, 546. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050546
Ciszewski M, Chmielarz A, Szołomicki Z, Drzazga M, Leszczyńska-Sejda K. Lead Recovery from Solid Residues of Copper Industry Using Triethylenetetramine Solution. Minerals. 2021; 11(5):546. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050546
Chicago/Turabian StyleCiszewski, Mateusz, Andrzej Chmielarz, Zbigniew Szołomicki, Michał Drzazga, and Katarzyna Leszczyńska-Sejda. 2021. "Lead Recovery from Solid Residues of Copper Industry Using Triethylenetetramine Solution" Minerals 11, no. 5: 546. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050546






