4.1. Similar Structures in Previous Work
Moro et al. [
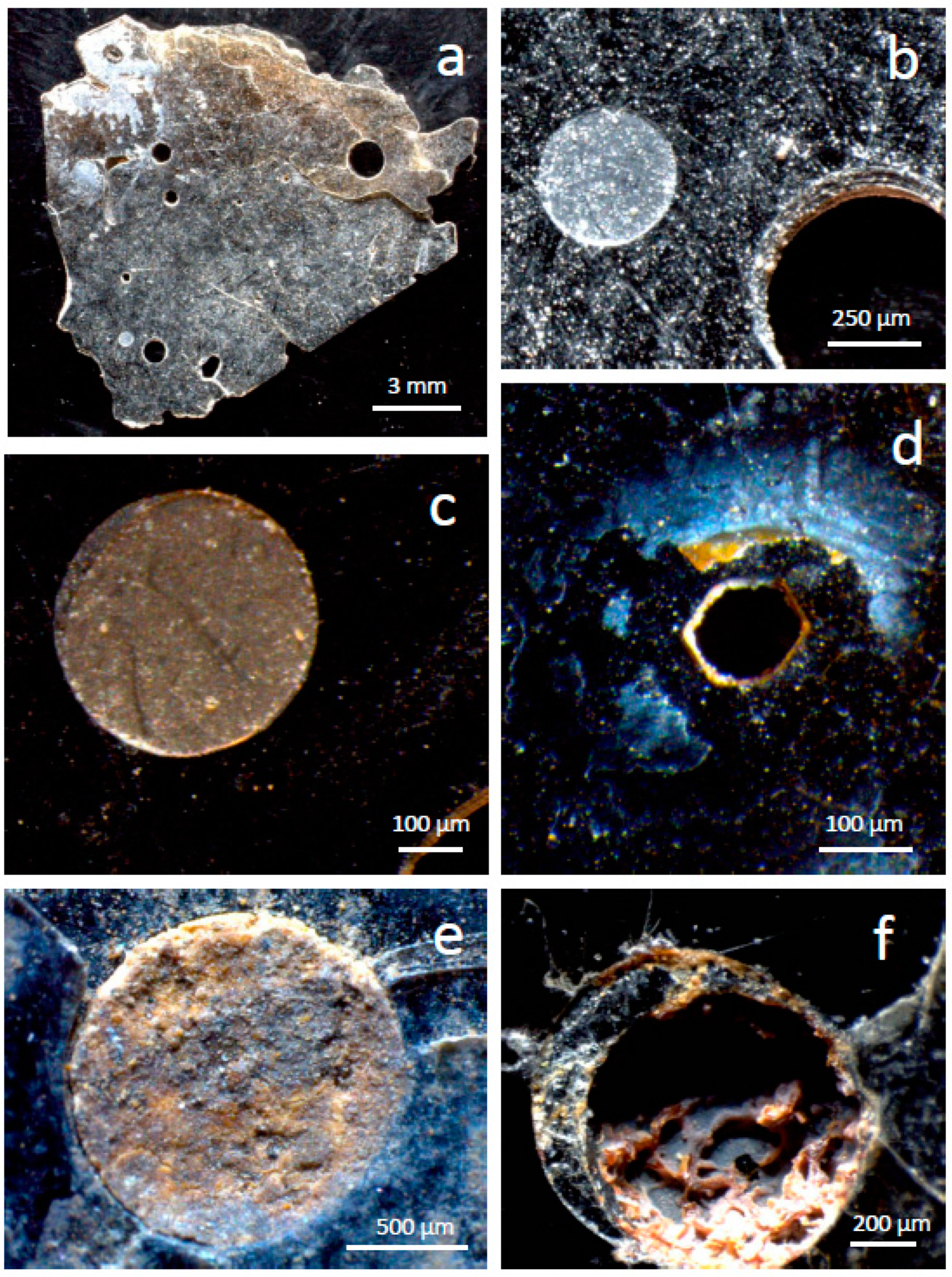
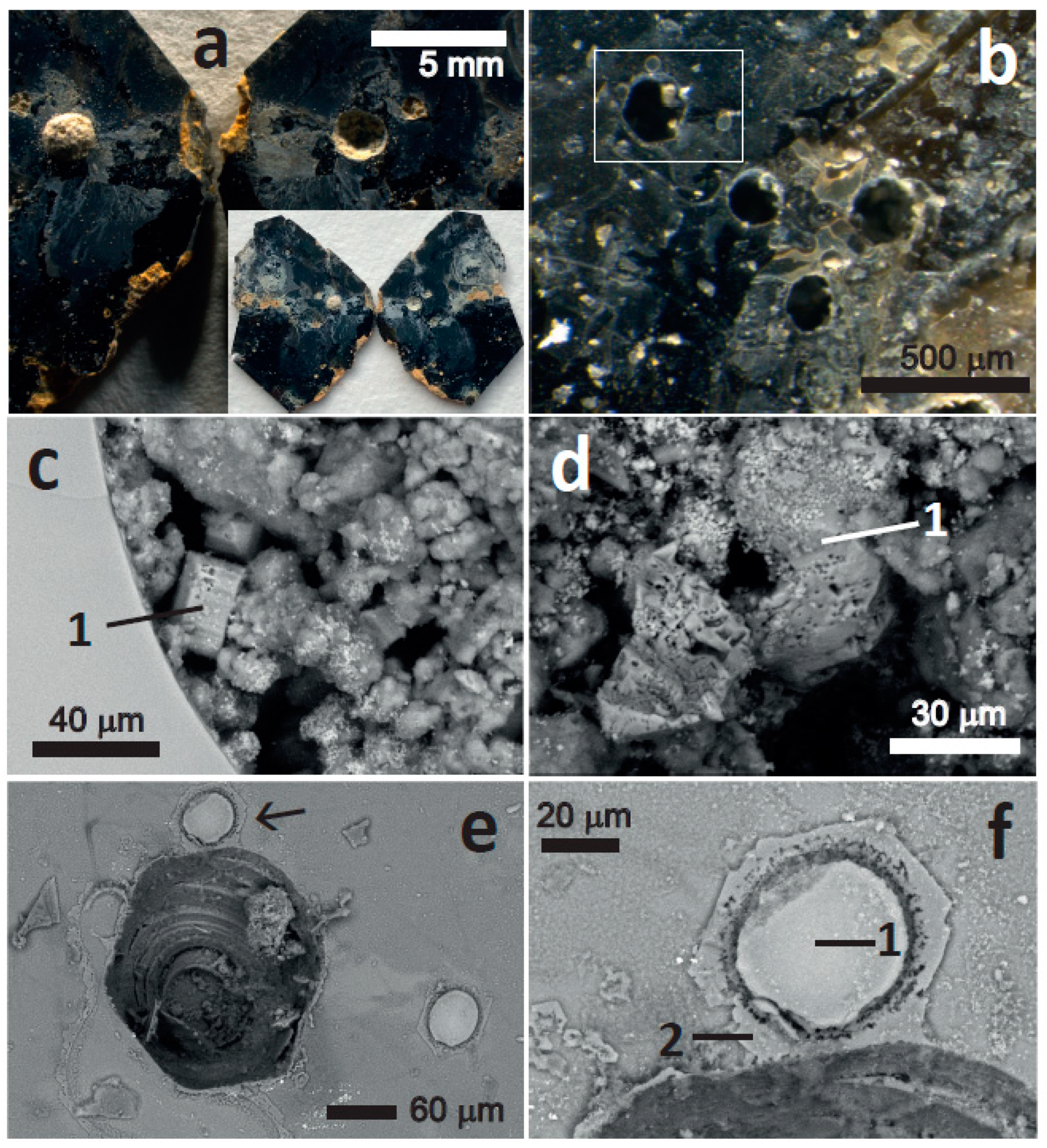
15] reported structures in a phlogopite from Uganda with similarities to the shallow depressions in our study (e.g.,
Figure 2c), consisting of depressions with internal steps. Shapes varied from near circular to very elongated and their size was smaller (a few nm to several μm) than the shallow depressions in the present study (hundreds of μm). The edges and steps of the depressions were entirely flat, with no sediment on them. From the evidence of bubbles of similar size and shapes containing hydrocarbons, Moro et al. [
15] concluded that the depressions were the interior of gas inclusions (bubbles) that had been exposed by crystal exfoliation.
Ghabru et al. [
16] described deep holes in altered biotite that they interpreted as due to mineral inclusions where the inclusions had disappeared. In their case, alteration took place within soils (A to C horizons of Gray Luvisols) formed on glacial tills in Saskatchewan. The alteration produced vermiculite and interstratified biotite-vermiculite, where the only chemical change was K loss. Thus, alteration conditions were much milder than in Caldara di Manziana. Although the holes were not considered the product of alteration, Ghabru et al. [
16] observed enhanced alteration at the edges of these holes and surrounding areas. One of such structures displayed in Ghabru et al. [
16] has a polygonal contour, is ~80 μm wide and is much deeper than is wide. There are signs of alteration within the hole and some debris of unknown composition.
Kurganskaya and Luttge [
17] showed depressions on muscovite produced during dissolution experiments conducted at pH 9.4 and 155 °C. The depressions have terraces and their shapes are from elongated and ellipsoidal to pseudo-hexagonal. Their size, however, is below <1 μm, much smaller than those in phlogopite from Caldara di Manziana. Kurganskaya and Luttge [
17] showed minute and dispersed particles within and around the depressions, but because their study was performed using AFM, it is not possible to know whether these particles were part of a precipitate deposited by dissolution.
None of the three cases is a good match for the structures and environmental conditions of the samples investigated here. The acidic alteration environment and the alteration products found within the structures in phlogopite from Caldara di Manziana are absent in the Ugandan and Canadian cases. The muscovite investigated by Kurganskaya and Luttge [
17] was not in an acidic medium, but it is possible that very thin precipitates were present.
4.2. Acidic Chemical Alteration
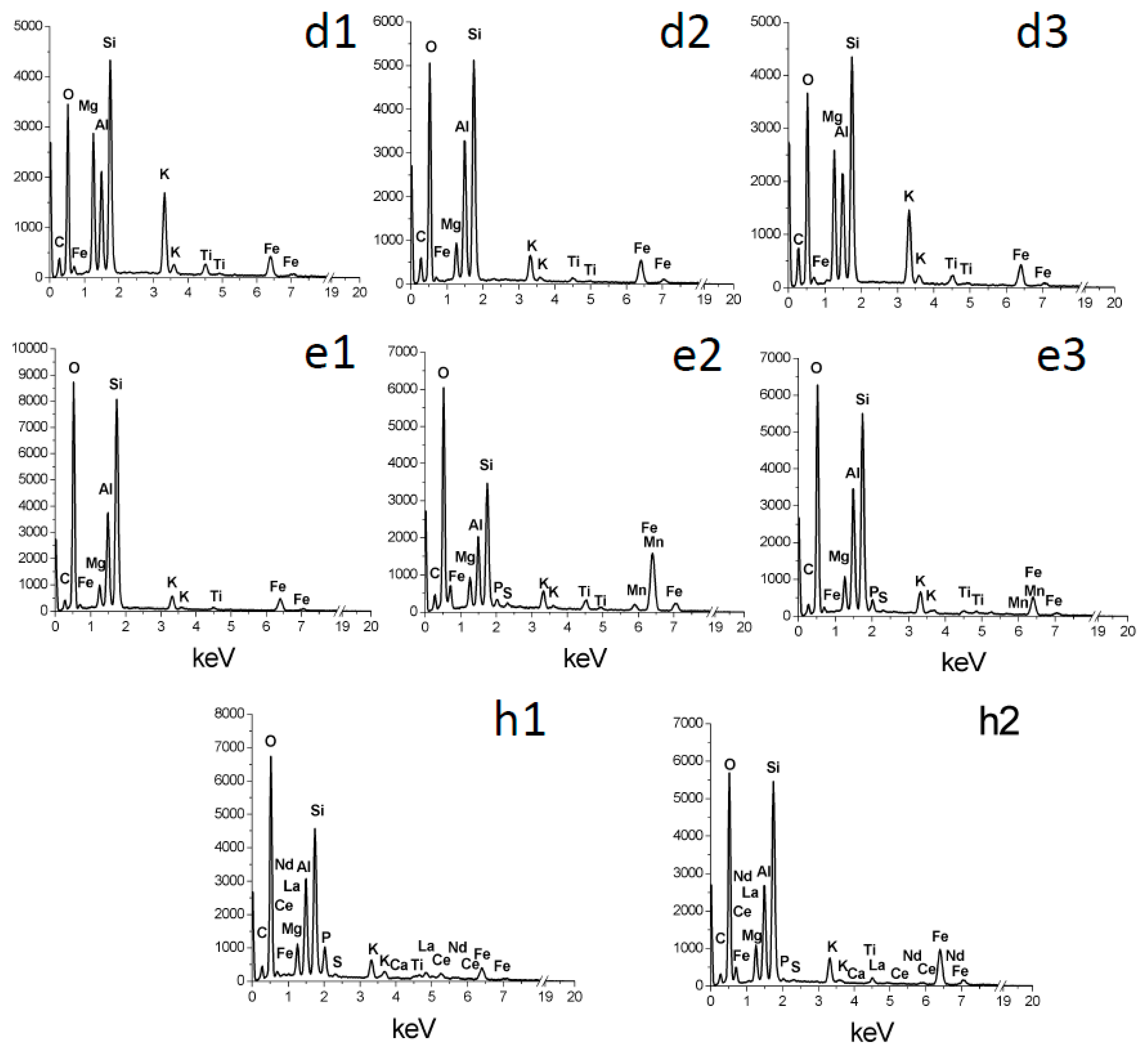
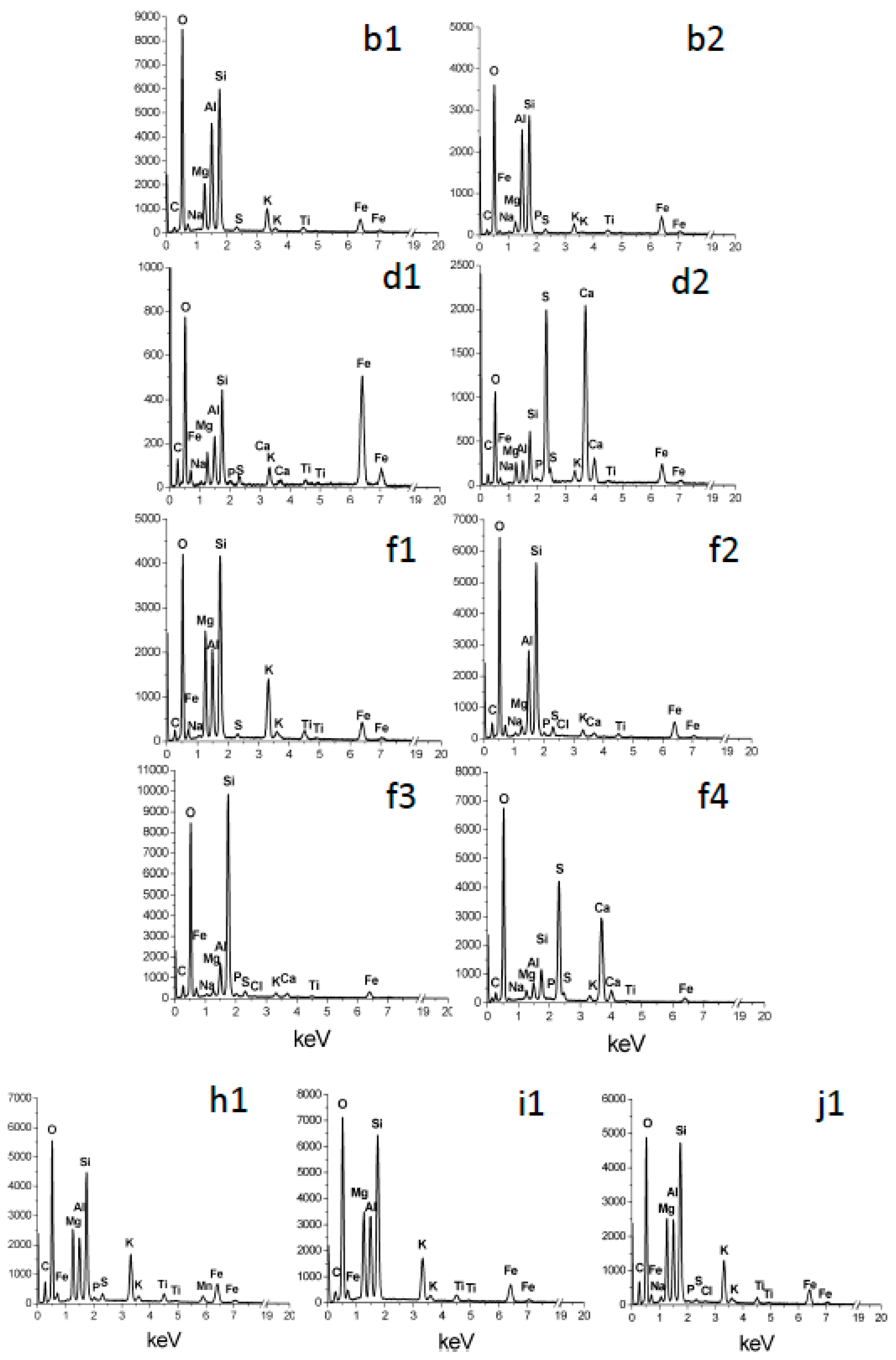
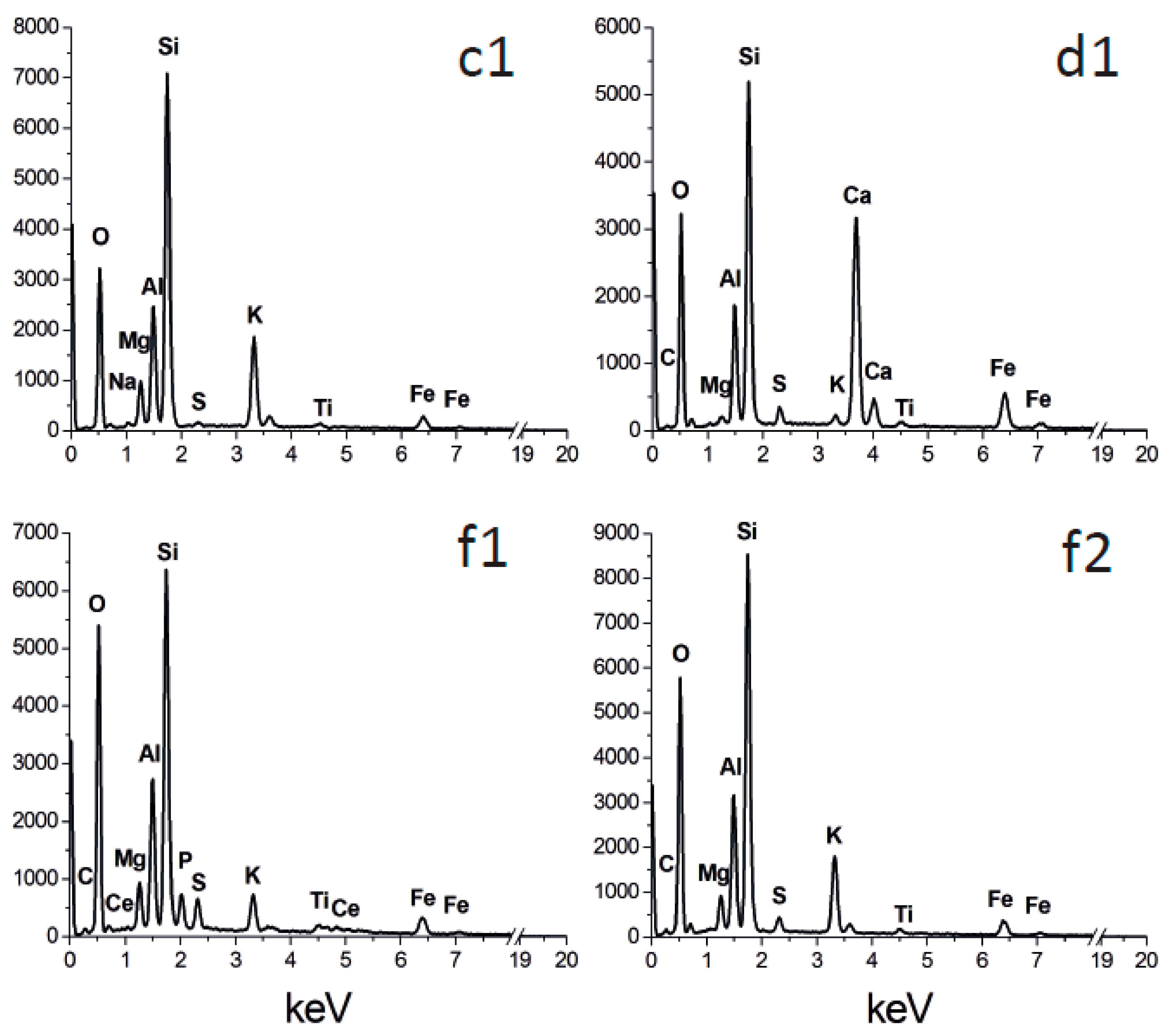
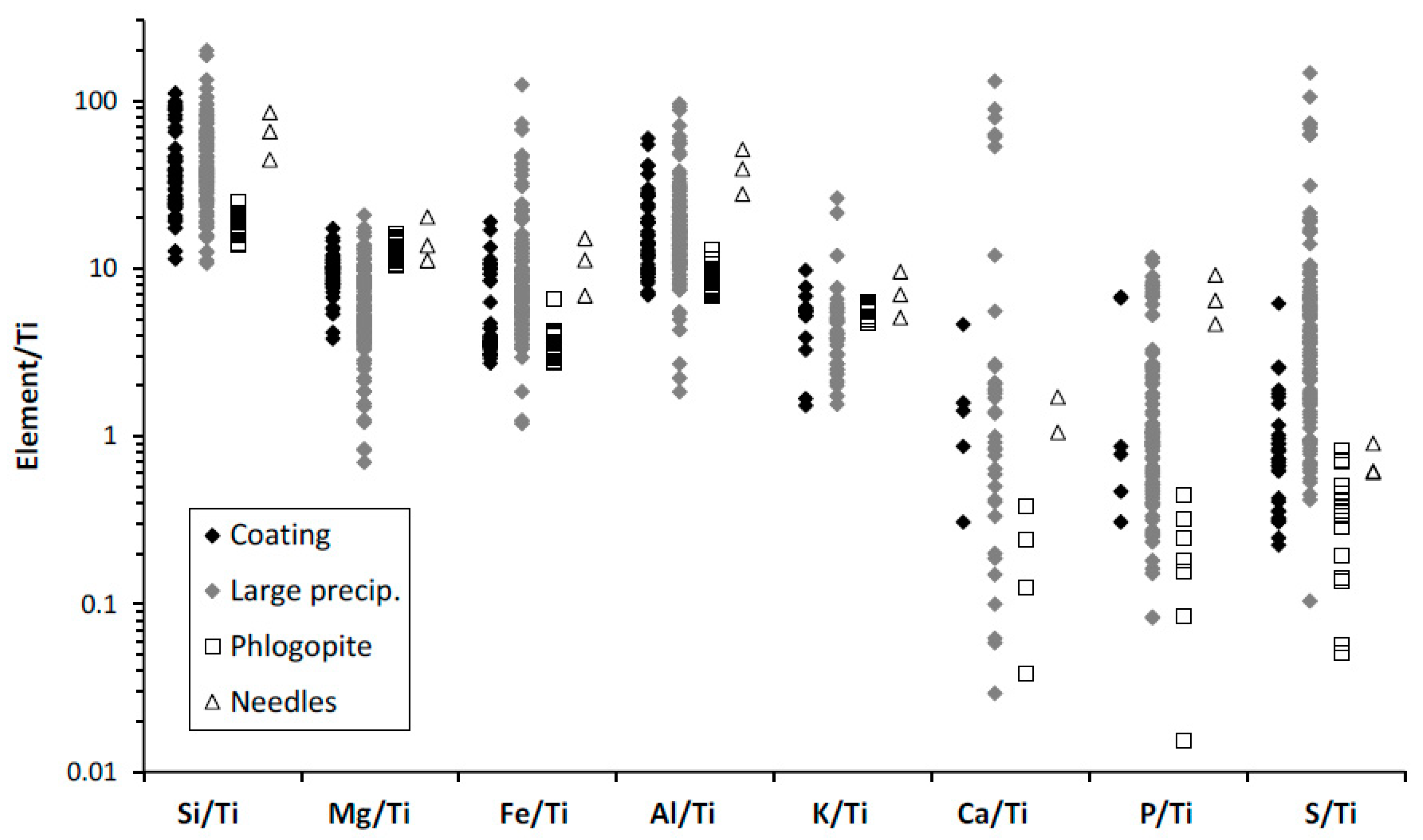
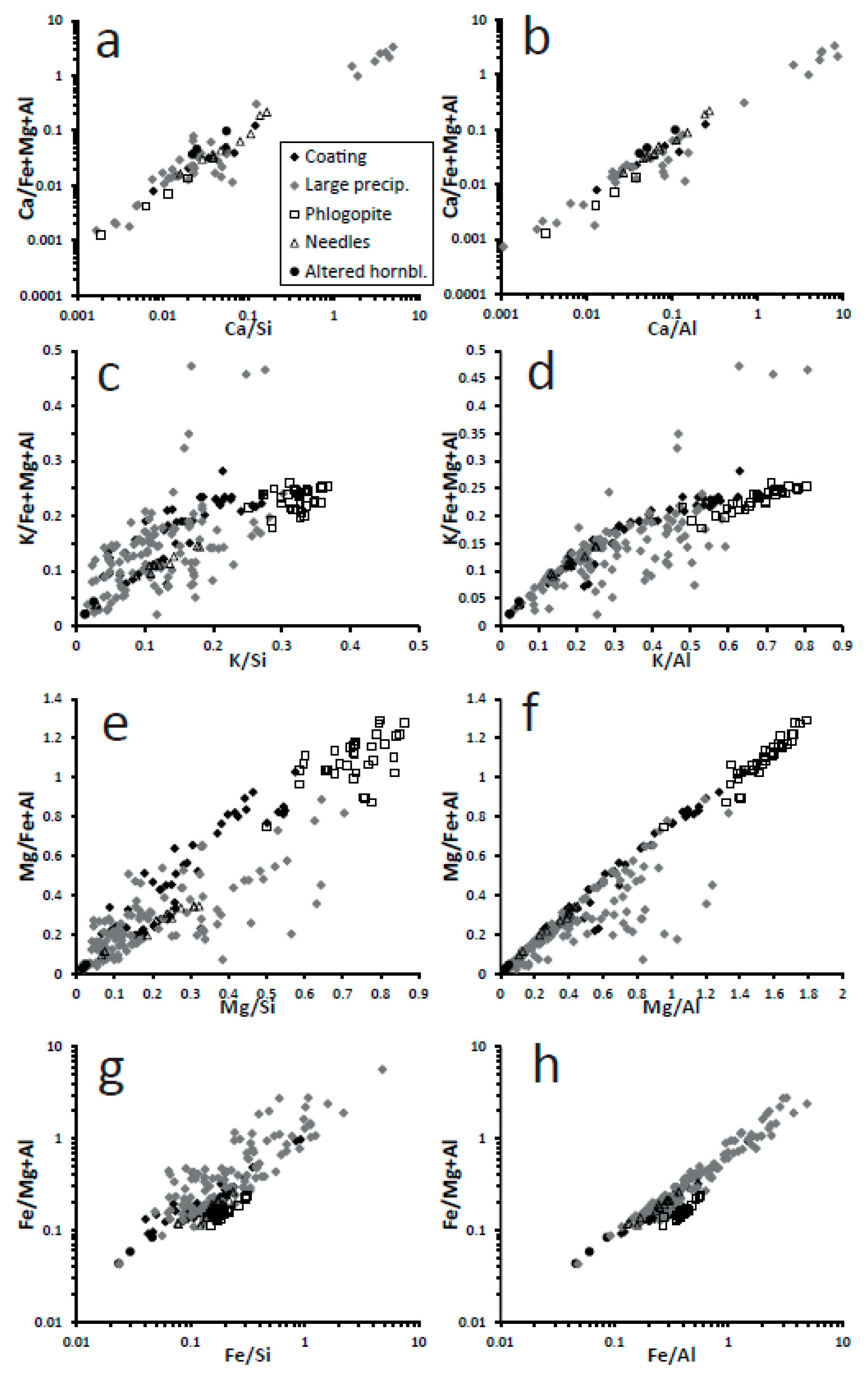
The overall chemical trends are congruent with the alteration of phlogopite taking place by acid attack. There is loss of K and Mg, two highly soluble cations, whereas there is concentration of Si, Al, and Fe (
Figure 10 and
Figure 11). Iron is less enriched than Si and Al in many cases (
Figure 11g,h). These are typical trends of acidic alteration. Kuwahara and Aoki [
18] dissolved phlogopite experimentally in 0.01 N HCl. Experiments longer than 6 days, where precipitation products first appeared, reproduced the pattern of cation loss in phlogopite from Caldara di Manziana. Iron- and Mg-rich silicates evolve towards Al silicates when subjected to acid alteration, although not exclusively in such conditions. Kaolinite and halloysite are produced from the alteration of more Mg- and Fe-rich silicates in acidic environments [
19,
20,
21] while beidellite is possibly a transient product in similar conditions. The thin deposits within depressions and steps in holes have a darker contrast in back-scattered electron images than that of the fresh phlogopite, coherent with a more hydrated composition (
Figure 2c,d,e), perhaps containing smectite and/or poorly crystallized, hydrated products. These sediments contain substantial residual Mg (
Figure 3d2,e1,e2,e3), indicating incomplete alteration.
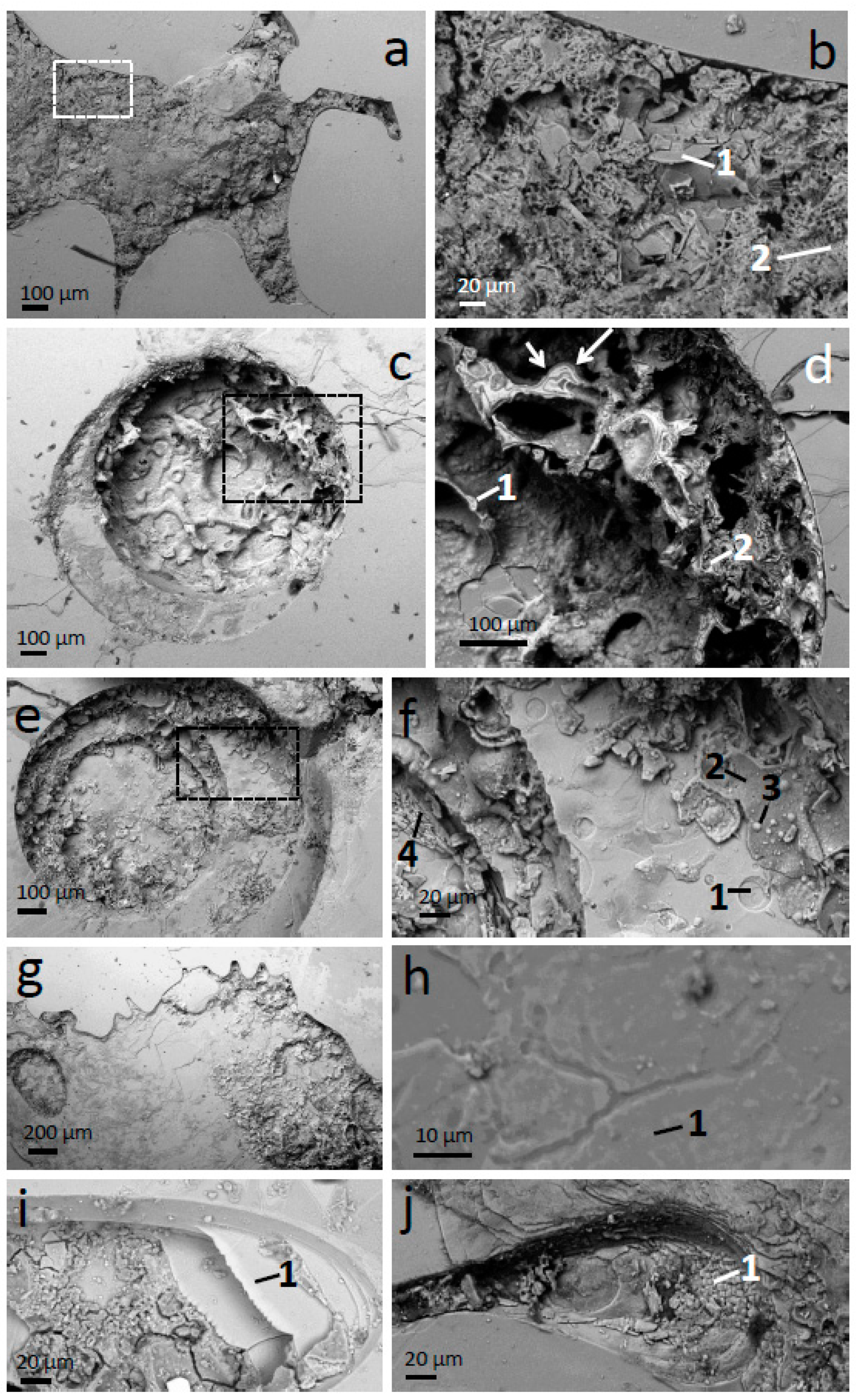
Figure 4b displays two very interesting morphologies, one platy, which can be interpreted as broken-up and partially altered phlogopite plates, still containing much Mg (
Figure 5b1), and a mesh of tubular structures with high Al content (
Figure 5b2). The latter structure can be interpreted as a silicate containing mainly Si and Al and some K, Fe, and Mg, which could be defined as beidellite. However, the tubular structures are suggestive of halloysite [
22], and it can be interpreted that the Mg, K, and Fe are not part of this mineral phase, but contamination of adjacent minerals (e.g., the plates, Fe oxides). It is, however, also possible that Mg, K, and Fe are in halloysite because halloysite and kaolinite are known to incorporate Fe, Mg, and interlayer cations, either within them or as interstratified smectite layers [
22,
23,
24,
25]. In any case, the tubes can be safely interpreted as an advanced alteration stage of the original phlogopite.
The coexistence of fresh phlogopite and intensely altered products (
Figure 4 and
Figure 5) supports an aggressive, intense and localized process, which is more coherent with acid attack than with advanced weathering at near neutral pH. Together with halloysite or beidellite, Fe oxides are also a product of acid attack of Fe-bearing silicates. Particularly, in phlogopite and biotite early disruption of the silicate crystal lattice causes Fe
2+ oxidization and release of Fe
3+ [
26]. Silica is a typical product of intense acid alteration [
27]. Gypsum is indicative of sulphate abundance, a natural residuum of sulphuric acid attack [
28]. The pseudo-hexagonal films suggestive of jarosite (
Figure 6e,f and
Figure 7f2) are also compatible with a localized acidic environment.
The three data points corresponding to altered hornblende follow almost the same chemical patterns (loss of Mg, Ca, and Fe; concentration of Si and Al) and indicate also acidic alteration (
Figure 11). Thus, the global chemical evolution of the alteration products, the composition of each specific alteration product and their morphologies all indicate acidic alteration.
4.3. Generation of Sulphuric Acid
The two major acidic gases emanated at Caldara di Manziana are, in the order of emanation rate, CO
2 and H
2S [
1,
6]. Both are mild acids when dissolved in water and unlikely to produce the focused and intense alteration features detected in the present investigation. The existence of elemental sulphur in Caldara di Manziana [
7] and the detection of sulphate within alteration cavities in phlogopite (gypsum:
Figure 4d,f and
Figure 5d2,f4) indicate that sulphur-oxidizing bacteria are causing oxidation of S
2− to S
0 and SO
42−. Sulphur-oxidizing bacteria are at the base of the microbial dissimilatory S cycle. They obtain metabolic energy from oxidizing reduced forms of S in a variety of compounds [
29]. Sulphuric acid is then the likely agent that caused the alteration. Shallow depressions with thin coatings were probably produced by diluted or small quantities of acid, whereas deep depressions and holes were produced by larger volumes of concentrated acid. This is consistent with gypsum being observed only in areas of intense alteration and large deposits of sediment, because gypsum would not precipitate from dilute solutions of sulphuric acid (gypsum solubility is ~2 g L
−1 at 25 °C [
30]).
The phenomenon of bacterial production of H
2SO
4 from H
2S followed by corrosion of steel and concrete elements is well documented in sewages and wastewater treatment facilities, where H
2S is the previous result of bacterial activity on the waste (e.g., [
31]). The process of biogenic sulphuric acid corrosion is driven by a succession of different bacterial species requiring different environmental pH [
32]. Pipes and exposed elements are first colonized by neutrophilic sulphur-oxidizing bacteria, such as
Thiobacillus spp. and
Thiomonas spp., which generate sulphuric acid and polythionic acids, lowering the pH to 3.5–5.0. As the pH decreases, other bacteria take over, in successive steps, which are capable of living at progressively lower pH, such as
Acidithiobacillus thiooxidans, and continue the process of sulphur oxidation and sulphuric acid production from pH 5 to as low as 1–2 [
31,
33].
It is of particular interest that the genus
Acidithiobacillus includes chemolithoautotrophic obligate acidophiles bacteria that can live with elemental sulphur as the only nutrient, producing high amounts of sulphuric acid [
34]. After other sulphur-oxidizing bacteria have generated S
0,
Acidithiobacillus spp. can generate sulphuric acid from this solid S source, meaning that a continuous acid attack of the mineral substrate can take place around the precipitated S
0 until it is consumed. An example of species that can accumulate solid S
0 that may be especially relevant to this study is that of chemolithotrophic sulphide- and sulphur-oxidizing bacteria (both types oxidize S, although following different biochemical paths). Their optimal habitats are narrow crevices, 0.1–1 mm wide, with low concentrations of dissolved H
2S and O
2 [
35], as it may be the case between the sheets of partially dislodged phlogopite crystals buried in the soil.
All the above-mentioned bacteria live in natural environments with abundant sources of sulphur, including soils [
8]. It can be reasonably concluded that the soils of Caldara di Manziana are the source of sulphuric acid by effect of the H
2S emanations and sulphur-oxidizing bacteria, and that the sulphuric acid corrodes and alters mineral grains in the soil.
4.4. Model of Phlogopite Acidic Alteration
Water is an essential element of the acidic alteration taking place in Caldara di Manziana because the sulphuric acid is required to be dissolved in water. Caldara di Manziana has a monthly average precipitation ranging from 40 mm, in July, to 171 mm, in November. The minimum and maximum average relative humidity occur in the same months and are 66 and 80%. Annual average precipitation and relative humidity are 99 mm and 74% [
36]. Thus, the climate in the area is rather humid. In addition, precipitation in the wettest season causes flooding of the centre of the caldera and water streams appear, while the focused CO
2 expulsion from the permanent water pool generates a continuous geyser and spray, all of which contribute to a still higher local relative humidity. As a consequence, minerals in the soil are expected to be frequently wet from rain or atmospheric condensation, and water droplets or films to remain long times on the mineral surfaces before evaporation. These are appropriate conditions for (1) H
2S to dissolve in the water and be available to sulphur-oxidizing bacteria and (2) biogenic sulphuric acid to dissolve in the water and attack surfaces in contact with it. Both steps of the process can take place frequently and for extended periods of time in Caldara di Manziana.
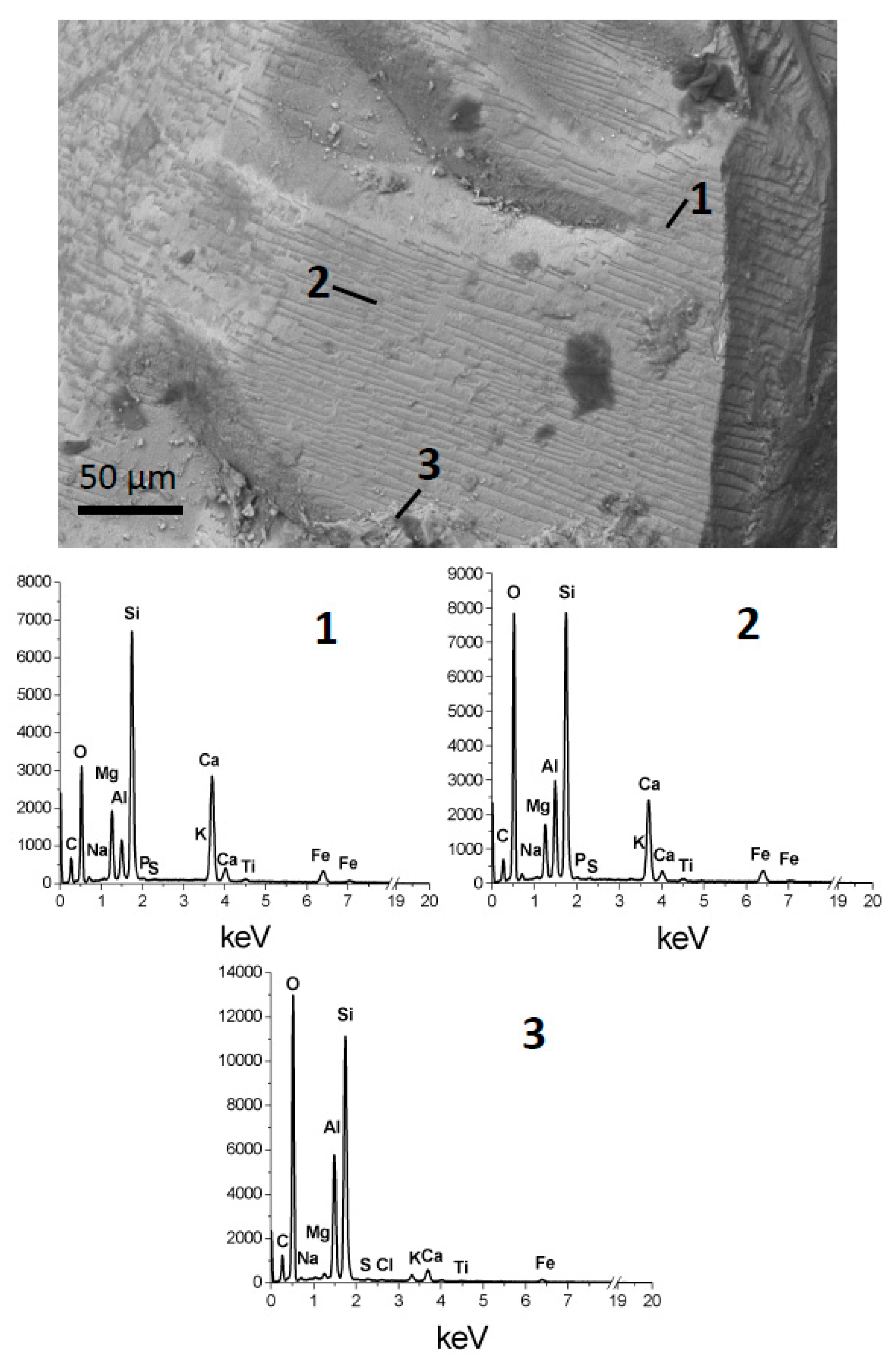
The altered material within depressions in the phlogopite crystals may have been produced in situ on the mineral grains or transported from surrounding altered grains in the soil. For example, hornblende phenocrysts in the soil have signs of acidic alteration (
Figure 8), indicating that acid attack does not take place exclusively on phlogopite and that translocation of alteration products was possible. Nonetheless, based on the following arguments, the alteration products found on phlogopite are interpreted to correspond to phlogopite alteration. First, shallow depressions had thin precipitates of clay-size flakes (
Figure 2) of a silicate composition (
Figure 3d2,e1,e2,e3), whereas deep depressions had thick precipitates (
Figure 4) covering a wide range of composition and morphology, including Fe oxides and silica (
Figure 5b1,b2,d1,d2,f2,f3,f4). Therefore, the intensity of the alteration is correlated with both the amount of material dissolved/altered/precipitated and its alteration stage, which is consistent with the precipitates forming largely in situ. Second, the altered material in phlogopite and hornblende crystals have a distinctive chemical character. Although both fall within the same chemical-ratio trends (
Figure 11), altered hornblende contains Ca (
Figure 8), which is not the case of altered phlogopite. This suggests that the altered material found on each mineral originated from the particular mineral. Calcium was found sometimes on altered phlogopite as gypsum (
Figure 5d2,f4) and in much smaller concentration as Ca phosphate (
Figure 9a). The existence of gypsum is indicative of Ca ions diffusing from the surrounding soil and precipitating with sulphate in areas where strong acid attack was taking place. There is also evidence of the transport and deposition of some foreign particles within deep depressions in phlogopite (K feldspar and hornblende in
Figure 6c,d). Thus, some solids and solutes were transported from surrounding soil to the altered phlogopite surfaces, but the evidence indicates them to be a minor component of the sediments on the mica.
The model of phlogopite alteration is interpreted to be the following. Emanating H
2S is dissolved in water on the surface or within partially cleaved crystals of phlogopite, where sulphur-oxidation bacteria oxidize sulphur to H
2SO
4 (
Figure 12) progressively and in stages. At some point, the sulphuric acid is sufficiently concentrated in droplets or water films to corrode the mineral. Shallow depressions (
Figure 1b,c and
Figure 2a–c,e) and some holes (
Figure 2d) produced by either attack on very thin flakes or exfoliation of flakes with shallow depressions are the result of acid attack of droplets with diluted sulphuric acid (left path in
Figure 12a). These droplets dissolve small volumes of phlogopite (shallow depressions) and leave a precipitate consisting of a fine-sized silicate and/or amorphous material with low-mobility cations. Proof that the acid concentration is low is that no sulphates or soluble substances precipitate in such depressions. Although phlogopite has low resistance to acid attack [
37] effective corrosion by dilute acid solutions requires a sustained period of attack, where water droplets are supposedly maintained by condensation of surrounding moisture. Moreover, consumed acid may need to be replaced, perhaps by diffusion from surrounding areas. Alternatively, micro-grains of S
0 precipitated by sulphur-oxidizing bacteria may be further oxidized to H
2SO
4 in the presence of water by mediation of other sulphur-oxidizing bacteria. The acid would then corrode the phlogopite surface in situ as new acid continues to be generated. This possibility provides the necessary replacement of acid to sustain the attack. The depth of the depression is controlled by the length of the attack, while the length of the attack is controlled by the availability of sulphuric acid. Acid availability can depend on a number of factors: proximity to a source of H
2S, concentration of sulphur-oxidation bacteria, concentration of S
0 grains, and length of the life of droplets or films where the acid is active. All of these factors are represented generically by H
2S availability in
Figure 12, where low availability results in a shallow depression (left path of
Figure 12a), and high availability (but always resulting in dilute acid concentration), producing deeper depressions and eventually a hole (right path in
Figure 12a).
High concentration of sulphuric acid in water droplets and films generates deep depressions and holes, where there are abundant precipitates with a wide range of compositions (partially altered phlogopite, beidellite or Fe,K-halloysite, silica, Fe oxides, and gypsum;
Figure 12c,d). Some debris from the surrounding soil may also be deposited in depressions and the phlogopite surface (
Figure 12d). The shapes of these cavities are variable and indicate different amount of acidic water as well as different interaction acid-phlogopite surface due to variables such as fluid-solid contact angle, phlogopite topography or angle of inclination of the mineral flakes. Some of such depressions are created by drops (
Figure 1,
Figure 2a–e,
Figure 4c,e and
Figure 6a–c,e,f), some by drops with a trail (
Figure 4j), some by advancing water corroding walls in different fashions perhaps following areas of weaker structure (
Figure 4a,g). High acid concentration and large volume of acid (i.e., large droplets and extended films) both promote longer attack. Conversely, these three factors, i.e., more aggressive acid, larger amount of acidic fluid, and longer life of the attack, cause greater irregularity of the corroded areas.
It is interesting that the shape of depressions or holes produced by droplets ranges from rounded to pseudo-hexagonal (
Figure 1 and
Figure 6). It is interpreted that rounded structures reflect the shape of the droplet on the phlogopite surface and indicate that the acidic attack took place similarly in every direction. Pseudo-hexagonal structures were created where the line of attack was controlled by the crystal symmetry of the mica. The condition for such a control is that the acid is not overly aggressive. In the same way as slow crystallization of phyllosilicates produces pseudo-hexagonal plates, slow dissolution produces pseudo-hexagonal cavities. Kurganskaya and Luttge [
17] show hexagonal to pseudo-hexagonal dissolution pits on the basal planes of muscovite. Slow growth and slow dissolution of minerals are controlled by the same principle: the crystallographic faces manifested are those containing the strongest bonds, according to the theory of periodic bond chains [
17]. The conditions within a depression may have changed over time, because the acid became more concentrated with time, as it dissolved the crystal deeper down, or because the dissolution of the phlogopite surface was a different event from the dissolution deep below the surface (e.g., a different cycle of water deposition and production or accumulation of sulphuric acid). This may be the reason why some depressions modified their shape with depth from pseudo-hexagonal to rounded (
Figure 6e).
The formation of approximately concentric terraces is a common dissolution phenomenon of phyllosilicates that form large crystals because they have a marked crystal anisotropy between the
c* axis and the plane containing the
a and
b axes (they are structures of piled sheets) and the dissolution is faster within the
ab plane than in the
c* direction [
17,
18,
38,
39]. Dissolution starts at weak points, such as dislocations, on the phlogopite surface. As dissolution proceeds faster laterally (
ab plane) than vertically (
c* direction) the formation of terraces is a natural phenomenon. The actual size and shape of the terraces is dependent on the intensity of the attack (in the present case, acid concentration), concentration of crystal defects, composition of the phyllosilicate, and protecting coating formed by precipitation, all of which modify the relative velocity of mica dissolution in one and the other direction. Generally, it would be expected that dilute acid generates wide terraces because the dissolution rate in the
ab plane is faster than in the
c* direction and the acid within the water droplet is consumed relatively quickly, so that the initial dissolution structure is preserved. Concentrated acid, however, will probably generate a chaotic morphology where terraces are absent in some places or have very different shapes, as controlled by changing conditions due to the fast dissolution and precipitation reactions [
39] and to modification of sulphuric acid concentration. This is coherent with the well-defined and relatively wide terraces observed in depressions and holes generated with diluted acid (
Figure 2) and with the more chaotic structures found where concentrated acid corroded phlogopite (
Figure 4 and
Figure 6).
It is possible that the type of alteration represented in
Figure 12d is the most common in phlogopite, i.e., that the conditions for the formation of depressions are most frequently met on the surfaces of partly cleaved crystals, but that the large majority of such crystals break up completely into two or more exfoliated flakes with depressions and holes of different character. Water within these partly cleaved crystals would evaporate slowly and allow for a sustained presence of sulphuric acid. There is, however, evidence that acidic alteration takes place on external mineral surfaces, as shown by altered hornblende, where attack on the outer surface is the only possibility. In these cases, contact with other mineral grains from the soil (in the surface of the soil or below it) may be also a mechanism to generate stable water droplets where acidic alteration may be sustained.
4.5. Unusual Dissolution and Precipitation Features
The SEM images of altered phlogopite display a group of unusual features.
Figure 4i shows a strongly altered area which is partly a depression and partly a hole. The depression is filled with a thick layer of alteration products with micro-cracks. Interestingly, some layers of phlogopite did not dissolve completely even if other layers between them did. The result is the building up of terraces of fairly pristine phlogopite (
Figure 5i1) separated by alteration products or with nothing in between them. It is surprising that the acid attacked and consumed phlogopite layers above and below and did not attack these portions that form the terraces. A related structure is present in
Figure 4j, where scales of fairly pristine phlogopite exist (
Figure 5j1). The scale structures appear to be at several heights (going higher up from left to right) and connected, creating a system of terraces with the appearance of scales. The scale appearance is generated by the fact that the terraces terminate in angles suggestive of a pseudo-hexagonal shape (two to four angles of ~120° are observed in many of the scales; there are several scales in each terrace). This structure must have been created by the partial dissolution of phlogopite layers, where the edges dissolved following the 120° angles of the planar symmetry of phlogopite (angles between two 110 crystallographic faces and between 110 and 010 faces). The scales are then another example of acid dissolution controlled by the phlogopite internal symmetry. The terrace and scale-like structures are indicative of very inhomogeneous acid alteration within the affected areas, because in the same depression there is, on the one hand, complete dissolution of phlogopite and large precipitates and, on the other, remains of phlogopite layers of almost pristine composition and showing slow dissolution controlled by crystal symmetry.
Perhaps a related path of strong (but always localized) alteration of phlogopite may be that represented in
Figure 4a,b, where phlogopite plates in advanced state of alteration (
Figure 5b1) coexist with tubes of halloysite or beidellite (
Figure 5b2). The plates appear to be broken up into pieces, as if one or several large plate-like structures had collapsed. There seems to be physical continuity between some plates and tubes, as if further alteration of the plates generates the tubes. This suggests the existence of large platy structures within the depression that have been subsequently altered into the mass of plates and tubes observed in
Figure 4a,b. It is interesting also that the top, right branch of the depression in
Figure 4a has an upper outline of three straight lines with angles of ~120°, and a more rounded outline at the bottom. This channel structure manifests heterogeneous dissolution conditions at distances < 100 μm, across the corresponding channel and between the channel and the rest of the depression.
Another unusual feature, now corresponding to a precipitation process, is the generation of hexagonal films around deep depressions. Such large structures (~50 μm across) are in
Figure 6e,f, but much smaller pseudo-hexagonal films are also observed in the left, bottom area of
Figure 6f. These films are interpreted to consist of jarosite, based on the combined presence of S, K and Fe (
Figure 7f2) and the pseudo-hexagonal shape of the films. These films seem to have developed from the depression and grown radially outwards. They may have precipitated as sulphate, Fe
3+, and K ions diffused out of the depression during the acid dissolution. The crystal where these structures were found was partially cleaved, but both sides across the cleavage surface remained in contact. This may explain the existence of a water film between both sides where the ions could diffuse radially and precipitate generating a mineral film. If the mineral film consists of jarosite, then the water film around the depression must have had a pH < 3, at which jarosite is stable [
40]. Moreover, the diffusion and precipitation must have taken place slowly to allow the growth of a single, extended film with a crystallographically controlled shape. The existence of a low-pH water film within the cleavage is consistent with the signs of chemical alteration of the phlogopite under the jarosite film (
Figure 7f2). However, the proximity of the EDX analysis point to the material within the depression may have contaminated it (
Figure 6f).