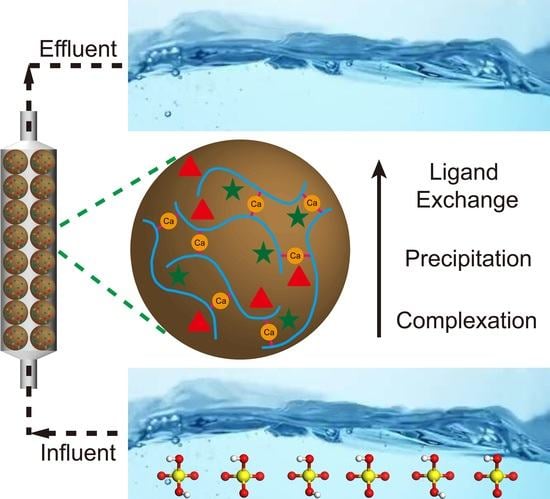
Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Powder Adsorbents
2.2.1. Synthesis of Mesoporous Silica Material
2.2.2. Synthesis of ALS
2.2.3. Synthesis of Magnetic Fe3O4
2.3. Synthesis of Hydrogel Bead Adsorbents
2.4. Characterization
2.5. Batch Experiments
2.6. Column Experiments
2.7. Analytical Methods
3. Results and Discussion
3.1. Characterization
3.2. Phosphate Adsorption by MALS-B
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
| Adsorbents | Concentration (mg/L) | pH | Temperature (°C) | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|
| Al-crosslinked PVA hydrogel beads | 0–110 | 6.0 | 30 | 26.0 | [34] |
| Al-inserted acid activated bentonite beads | 6–183 | 3.0 | 25 | 39.4 | [35] |
| MgFe2O4-BM-La(b) | 5–50 | 5.3 | 25 | 26.83 | [23] |
| Zr-modified chitosan beads | 5–50 | 4.0 | 25 | 60.6 | [36] |
| Fe-sericite beads | 3–61 | 5.0 | 25 | 13.6 | [37] |
| NH-SA-ZrBT | 5–200 | 7.0 | 25 | 63.9 | [22] |
| SA-ZrBT | 5–100 | 7.0 | 25 | 37.5 | [22] |
| Fe3O4/PAM/SA-Zr beads | 0–100 | 2.0 | 25 | 42.43 | [38] |
| ALS-B | 0–70 | 5.3 | 25 | 46.08 | This work |
| MALS-B | 0–70 | 5.3 | 25 | 40.14 | This work |
3.2.3. Effects of pH and Interfering Ions
3.2.4. Fixed-Bed Column Adsorption
3.3. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, C.; Klementiev, K.; Hassenkam, T.; Qian, W.; Ai, J.; Hansen, H.C.B. High affinity lanthanum doped iron oxide nanosheets for phosphate removal. Chem. Eng. J. 2021, 422, 130009. [Google Scholar] [CrossRef]
- Yang, W.; Shi, X.; Dong, H.; Tang, H.; Chen, W.; Wu, M.; Hua, M.; Zhang, W. Fabrication of a reusable polymer-based cerium hydroxide nanocomposite with high stability for preferable phosphate removal. Chem. Eng. J. 2021, 405, 126649. [Google Scholar] [CrossRef]
- Wan, J.; Wu, B.; Lo, I.M.C. Development of Fe0/Fe3O4 composites with tunable properties facilitated by Fe2+ for phosphate removal from river water. Chem. Eng. J. 2020, 388, 124242. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Liu, J.; Takahashi, M.; Tsubouchi, N. Adsorption of phosphate from aqueous using iron hydroxides prepared by various methods. J. Environ. Chem. Eng. 2021, 9, 104645. [Google Scholar] [CrossRef]
- Xing, B.; Ouyang, M.; Graham, N.; Yu, W. Enhancement of phosphate adsorption during mineral transformation of natural siderite induced by humic acid: Mechanism and application. Chem. Eng. J. 2020, 393, 124730. [Google Scholar] [CrossRef]
- Pan, J.; Gao, B.; Song, W.; Xu, X.; Yue, Q. Modified biogas residues as an eco-friendly and easily-recoverable biosorbent for nitrate and phosphate removals from surface water. J. Hazard. Mater. 2020, 382, 121073. [Google Scholar] [CrossRef]
- Liu, X.; Zong, E.; Hu, W.; Song, P.; Wang, J.; Liu, Q.; Ma, Z.; Fu, S. Lignin-derived porous carbon loaded with La(OH)3 nanorods for highly efficient removal of phosphate. ACS Sustain. Chem. Eng. 2018, 7, 758–768. [Google Scholar] [CrossRef]
- Kong, L.; Tian, Y.; Pang, Z.; Huang, X.; Li, M.; Yang, R.; Li, N.; Zhang, J.; Zuo, W. Synchronous phosphate and fluoride removal from water by 3D rice-like lanthanum-doped La@MgAl nanocomposites. Chem. Eng. J. 2019, 371, 893–902. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour. Technol. 2008, 99, 8685–8690. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Qi, Z.; Zhang, L.; Zhang, Y.; Huang, H.; Peng, Y. Designing ZIF-8/hydroxylated MWCNT nanocomposites for phosphate adsorption from water: Capability and mechanism. Chem. Eng. J. 2020, 394, 124992. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Wang, H.; Zhang, G.; Zhang, T.; Long, Z.; Xian, G. Advanced phosphate and nitrogen removal in water by La-Mg composite. Environ. Res. 2021, 193, 110529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, Y.; Yang, Y.; Zhang, Y.; Lei, X.; Yuan, D. Application of FeMgMn layered double hydroxides for phosphate anions adsorptive removal from water. Appl. Clay. Sci. 2021, 200, 105903. [Google Scholar] [CrossRef]
- Qu, J.; Akindolie, M.S.; Feng, Y.; Jiang, Z.; Zhang, G.; Jiang, Q.; Deng, F.; Cao, B.; Zhang, Y. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration. Chem. Eng. J. 2020, 394, 124915. [Google Scholar] [CrossRef]
- Huo, J.; Min, X.; Wang, Y. Zirconium-modified natural clays for phosphate removal: Effect of clay minerals. Environ. Res. 2021, 194, 110685. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Tang, H.; Jin, Z.; Mao, Y.; Huang, T. Removal of phosphate from wastewater by modified bentonite entrapped in Ca-alginate beads. J. Environ. Manag. 2020, 260, 110130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Chen, X.; Huang, C.; Yang, S. Biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles for phosphate adsorption. J. Hazard. Mater. 2020, 396, 122626. [Google Scholar] [CrossRef]
- Delgadillo-Velasco, L.; Hernández-Montoya, V.; Ramírez-Montoya, L.A.; Montes-Morán, M.A.; del Rosario Moreno-Virgen, M.; Rangel-Vázquez, N.A. Removal of phosphate and aluminum from water in single and binary systems using iron-modified carbons. J. Mol. Liq. 2021, 323, 114586. [Google Scholar] [CrossRef]
- Moraes Schambeck, C.; Ribeiro da Costa, R.H.; Derlon, N. Phosphate removal from municipal wastewater by alginate-like exopolymers hydrogels recovered from aerobic granular sludge. Bioresour. Technol. 2021, 333, 125167. [Google Scholar] [CrossRef]
- Shan, X.; Zhao, Y.; Bo, S.; Yang, L.; Xiao, Z.; An, Q.; Zhai, S. Magnetic aminated lignin/CeO2/Fe3O4 composites with tailored interfacial chemistry and affinity for selective phosphate removal. Sci. Total Environ. 2021, 796, 148984. [Google Scholar] [CrossRef]
- Ahmed, S.; Zhang, Y.; Wu, B.; Zheng, Z.; Leung, C.F.; Choy, T.Y.; Kwok, Y.T.; Lo, I.M.C. Scaled-up development of magnetically recyclable Fe3O4/La(OH)3 composite for river water phosphate removal: From bench-scale to pilot-scale study. Sci. Total Environ. 2021, 791, 148281. [Google Scholar] [CrossRef]
- Ahmed, S.; Lo, I.M.C. Phosphate removal from river water using a highly efficient magnetically recyclable Fe3O4/La(OH)3 nanocomposite. Chemosphere 2020, 261, 128118. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly effective removal of phosphate from complex water environment with porous Zr-bentonite alginate hydrogel beads: Facile synthesis and adsorption behavior study. Appl. Clay. Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yan, W.; He, C.; Shi, Y. MgFe2O4-biochar based lanthanum alginate beads for advanced phosphate removal. Chem. Eng. J. 2020, 387, 123305. [Google Scholar] [CrossRef]
- Chen, H.; Yang, H.; Xi, Y. Highly ordered and hexagonal mesoporous silica materials with large specific surface from natural rectorite mineral. Microporous Mesoporous Mater. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Shan, S.; Tang, H.; Zhao, Y.; Wang, W.; Cui, F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem. Eng. J. 2019, 359, 779–789. [Google Scholar] [CrossRef]
- Jung, K.W.; Jeong, T.U.; Kang, H.J.; Ahn, K.H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, H.; Zheng, K.; Zhang, Z.; Jiang, Q.; Li, J. Two-dimensional hydrophilic ZIF-L as a highly-selective adsorbent for rapid phosphate removal from wastewater. Sci. Total Environ. 2021, 785, 147382. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.; Yang, L. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2021, 322, 114523. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Wang, J.; Tan, Q.; Fu, L. Utilization of iron tailings to prepare high-surface area mesoporous silica materials. Sci. Total Environ. 2020, 736, 139483. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, B.; Xu, H.; Chen, J.; Liu, Q.; Deng, Y.; Zeng, H. Highly regenerable mussel-inspired Fe3O4@polydopamine-Ag core-shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl. Mater. Interfaces 2014, 6, 8845–8852. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, D.; Yue, G.; Zhu, J.; Zhao, P. Ca2+ induced 3D porous MXene gel for continuous removal of phosphate and uranium. Appl. Surf. Sci. 2021, 570, 150804. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Zhang, Y.; Ye, L. Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem. Eng. J. 2014, 235, 207–214. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gupta, P.; Lalhmunsiama; Bajaj, H.C.; Lee, S.M. Al-intercalated acid activated bentonite beads for the removal of aqueous phosphate. Sci. Total Environ. 2016, 572, 1222–1230. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Lee, C.; Jung, J.; Pawar, R.R.; Kim, M.; Lalhmunsiama; Lee, S.-M. Arsenate and phosphate removal from water using Fe-sericite composite beads in batch and fixed-bed systems. J. Ind. Eng. Chem. 2017, 47, 375–383. [Google Scholar] [CrossRef]
- Luo, H.; Rong, H.; Zhang, T.C.; Zeng, X.; Wan, J. Amino-functionalized magnetic zirconium alginate beads for phosphate removal and recovery from aqueous solutions. J. Appl. Polym. Sci. 2018, 136, 46897. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.; Wu, Z.; Xu, F.; Yang, D.; He, Q.; Li, G.; Chen, Y. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresour. Technol. 2019, 289, 121600. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.; Tian, Y.; Sun, L.; Zhan, W.; Chen, S.; Zhang, J.; Zuo, W. Three-dimensional graphene/La(OH)3-nanorod aerogel adsorbent by self-assembly process for enhanced removal and recovery of phosphate in wastewater. Sci. Total Environ. 2021, 809, 152124. [Google Scholar] [CrossRef]
- Hao, H.; Wang, Y.; Shi, B. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study. Water Res. 2019, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Y.; Li, X.; Wang, Y. Investigation of phosphate removal mechanisms by a lanthanum hydroxide adsorbent using p-XRD, FTIR and XPS. Appl. Surf. Sci. 2021, 557, 149838. [Google Scholar] [CrossRef]
- Zong, E.; Huang, G.; Liu, X.; Lei, W.; Jiang, S.; Ma, Z.; Wang, J.; Song, P. A lignin-based nano-adsorbent for superfast and highly selective removal of phosphate. J. Mater. Chem. A 2018, 6, 9971–9983. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg-Al-La ternary (hydr)oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Wu, B.; Lo, I.M.C. Surface functional group engineering of CeO2 particles for enhanced phosphate adsorption. Environ. Sci. Technol. 2020, 54, 4601–4608. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, Z.; Chang, X.; Ma, G.; Meng, L.; Liu, T.; Yang, L.; Guo, Y.; Ma, X. Hollow hierarchically porous La2O3 with controllable multishells: A high-performance adsorbent for phosphate removal. Chem. Eng. J. 2021, 421, 127816. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Yang, H. Lanthanum compounds-modified rectorite composites for highly efficient phosphate removal from wastewater. Appl. Clay. Sci. 2020, 199, 105875. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La(III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.; Han, Q.; Lou, S.; Zhang, L.; Zhang, W. Fast and efficient phosphate removal on lanthanum-chitosan composite synthesized by controlling the amount of cross-linking agent. Int. J. Biol. Macromol. 2020, 157, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, G.; Long, Z.; Xian, G.; Niu, L. Advanced phosphate removal by La-Zr-Zn ternary oxide: Performance and mechanism. J. Alloys Compd. 2020, 817, 152745. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zeng, H.; Yang, H. Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads. Minerals 2022, 12, 171. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020171
Chen H, Zeng H, Yang H. Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads. Minerals. 2022; 12(2):171. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020171
Chicago/Turabian StyleChen, Hongyun, Hongbo Zeng, and Huaming Yang. 2022. "Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads" Minerals 12, no. 2: 171. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020171