Fabrication of Hydrotalcite-like Copper Hydroxyl Salts as a Photocatalyst and Adsorbent for Hexavalent Chromium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Hydrotalcite-like Copper Hydroxyl Salts
2.3. Characterization
2.4. Photocatalytic Test
3. Results
3.1. Characterizations
3.2. Hexavalent Chromium (Cr(VI) Removal
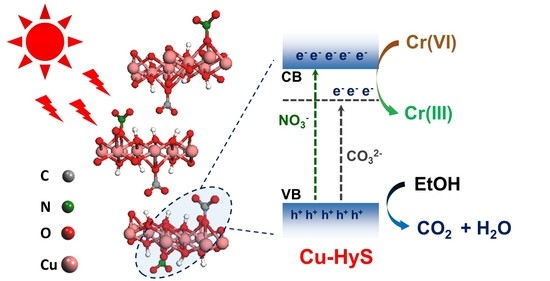
3.3. Verification of Charge Transfer, Separation and Production of Electron and Hole
3.4. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, N. Mechanochemical synthesis of layered hydroxy salts. Mater. Res. Bull. 2012, 47, 3568–3572. [Google Scholar] [CrossRef]
- Li, B.-C.; Huy, N.N.; Lin, J.-Y.; Phattarapattamawong, S.; Lisak, G.; Wang, H.; Lin, K.-Y.A. Nanopetal-like copper hydroxide nitrate as a highly selective heterogeneous catalyst for valorization of vanillic alcohol via oxidation. J. Environ. Chem. Eng. 2021, 9, 106092. [Google Scholar] [CrossRef]
- Srikhaow, A.; Smith, S.M.; Uraisin, K.; Suttiponparnit, K.; Kongmark, C.; Chuaicham, C. Catalytic remediation of phenol contaminated wastewater using Cu–Zn hydroxide nitrate. RSC Adv. 2016, 6, 36766–36774. [Google Scholar] [CrossRef]
- Weeramonkhonlert, V.; Srikhaow, A.; Smith, S.M. Formation of copper hydroxy double salts derived from metal oxides and their catalytic activity in degradation of methyl orange. Ceram. Int. 2019, 45, 993–1000. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Pan, L.; Zhao, F.-M.; Zou, J.-J.; Zhang, T.; Wang, L. Controllable sonochemical synthesis of Cu2O/Cu2(OH)3NO3 composites toward synergy of adsorption and photocatalysis. Appl. Catal. B Environ. 2015, 164, 234–240. [Google Scholar] [CrossRef]
- Anandan, S.; Wu, J.J.; Ashokkumar, M. Sonochemical Synthesis of Layered Copper Hydroxy Nitrate Nanosheets. ChemPhysChem 2015, 16, 3389–3391. [Google Scholar] [CrossRef]
- da Rocha, M.G.; Nakagaki, S.; Ucoski, G.M.; Wypych, F.; Sippel Machado, G. Comparison between catalytic activities of two zinc layered hydroxide salts in brilliant green organic dye bleaching. J. Colloid Interface Sci. 2019, 541, 425–433. [Google Scholar] [CrossRef]
- Dai, T.; Mao, Y. ZnO-assisted synthesis of multilayered Cu2(OH)3NO3 nanoplates and application removal of methyl orange. Chem. Phys. Lett. 2020, 739, 137018. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, X. Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2. Minerals 2020, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Henrist, C.; Traina, K.; Hubert, C.; Toussaint, G.; Rulmont, A.; Cloots, R. Study of the morphology of copper hydroxynitrate nanoplatelets obtained by controlled double jet precipitation and urea hydrolysis. J. Cryst. Growth 2003, 254, 176–187. [Google Scholar] [CrossRef]
- Liu, B. One-dimensional copper hydroxide nitrate nanorods and nanobelts for radiochemical applications. Nanoscale 2012, 4, 7194–7198. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, H.; Chen, R.; Wang, P.; Yang, H.; Gao, M.; Peng, X.; Ye, Z. Synthesis of CuO nanowalnuts and nanoribbons from aqueous solution and their catalytic and electrochemical properties. Nanoscale 2012, 4, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Srikhaow, A.; Smith, S.M. Preparation of Cu2(OH)3NO3/ZnO, a novel catalyst for methyl orange oxidation under ambient conditions. Appl. Catal. B Environ. 2013, 130–131, 84–92. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, copper, and copper hydroxy salt decorated fumed silica hybrid composites as antibacterial agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Manivannan, R.; Chuaicham, C.; Karthikeyan, S.; Sasaki, K. A simple tactic synthesis of hollow porous graphitic carbon nitride with significantly enhanced photocatalytic performance. Chem. Commun. 2021, 57, 6772–6775. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Ramalingam, M.; Sekar, K.; Chuaicham, C.; Sasaki, K. Fabrication and characterization of carbon quantum dots decorated hollow porous graphitic carbon nitride through polyaniline for photocatalysis. Chem. Eng. J. 2021, 426, 131739. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Ohtani, B.; Sasaki, K. Visible light-driven ZnCr double layer oxide photocatalyst composites with fly ashes for the degradation of ciprofloxacin. J. Environ. Chem. Eng. 2021, 10, 106970. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Xiong, Y.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Dabo, I.; Sasaki, K. Single-step synthesis of oxygen-doped hollow porous graphitic carbon nitride for photocatalytic ciprofloxacin decomposition. Chem. Eng. J. 2021, 425, 130502. [Google Scholar] [CrossRef]
- Chuaicham, C.; Xiong, Y.; Sekar, K.; Chen, W.; Zhang, L.; Ohtani, B.; Dabo, I.; Sasaki, K. A promising Zn-Ti layered double hydroxide/Fe-bearing montmorillonite composite as an efficient photocatalyst for Cr(VI) reduction: Insight into the role of Fe impurity in montmorillonite. Appl. Surf. Sci. 2021, 546, 148835. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Vellaichamy, B.; Li, W.; Zhuang, W.; Lu, X.; Ohtani, B.; Sasaki, K. Cubic Cu2O nanoparticles decorated on TiO2 nanofiber heterostructure as an excellent synergistic photocatalyst for H2 production and sulfamethoxazole degradation. Appl. Catal. B Environ. 2021, 294, 120221. [Google Scholar] [CrossRef]
- Pereira Rocha, R.L.; Silva, T.L.; Araujo, F.P.; Vieira, E.G.; Honório, L.M.; Furtini, M.B.; da Fonseca, M.G.; Silva-Filho, E.C.d.; Osajima, J.A. Gallium-Containing Hydroxyapatite as a Promising Material for Photocatalytic Performance. Minerals 2021, 11, 1347. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.-R.; Valmalette, J.-C.; Benlhachemi, A. Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B. Minerals 2021, 11, 1007. [Google Scholar] [CrossRef]
- Zhang, L.; Chuaicham, C.; Balakumar, V.; Ohtani, B.; Sasaki, K. Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol. Minerals 2021, 11, 1381. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Basitere, M. Interactive Relationship between Cementitious Materials and Acid Mine Drainage: Their Effects on Chromium Cr(VI) Removal. Minerals 2020, 10, 932. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, H.; Xiong, J.; Xu, S.; An, D. Z-scheme AgBr@Ag/CoAl layered double hydroxide heterojunction for superior photocatalytic Cr(VI) reduction under visible light. Appl. Clay Sci. 2020, 192, 105627. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, D.; Li, N.; Li, H.; Xu, Q.; He, J.; Lu, J. Construction of hollow In2S3/CdIn2S4 heterostructures with high efficiency for Cr(vi) reduction. Environ. Sci. Nano 2021, 8, 1389–1397. [Google Scholar] [CrossRef]
- Padhi, D.K.; Panigrahi, T.K.; Parida, K.; Singh, S.K.; Mishra, P.M. Green Synthesis of Fe3O4/RGO Nanocomposite with Enhanced Photocatalytic Performance for Cr(VI) Reduction, Phenol Degradation, and Antibacterial Activity. ACS Sustain. Chem. Eng. 2017, 5, 10551–10562. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Jing, Q.Y.; Chen, J.T.; Li, L. Photocatalytic Cr(VI) reduction by mixed metal oxide derived from ZnAl layered double hydroxide. Appl. Clay Sci. 2017, 143, 168–174. [Google Scholar] [CrossRef]
- Yuan, G.; Li, F.; Li, K.; Liu, J.; Li, J.; Zhang, S.; Jia, Q.; Zhang, H. Research Progress on Photocatalytic Reduction of Cr(VI) in Polluted Water. Bull. Chem. Soc. Jpn. 2020, 94, 1142–1155. [Google Scholar] [CrossRef]
- El-Taweel, Y.A.; Nassef, E.M.; Elkheriany, I.; Sayed, D. Removal of Cr(VI) ions from waste water by electrocoagulation using iron electrode. Egypt. J. Pet. 2015, 24, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, H.; Liu, F.; Yang, F.; Zhou, S.; Zheng, K.; Chu, C.; Liu, L.; Ju, M. Effective removal of Cr(vi) from aqueous solution by biochar supported manganese sulfide. RSC Adv. 2019, 9, 31333–31342. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, B.; Nguyen, T.N.; Kampouri, S.; Sun, D.T.; Mensi, M.D.; Stylianou, K.; Smit, B.; Queen, W.L. A novel integrated Cr(vi) adsorption–photoreduction system using MOF@polymer composite beads. J. Mater. Chem. A 2020, 8, 9629–9637. [Google Scholar] [CrossRef]
- Rahmani, A.r.; Hossieni, E.; Poormohammadi, A. Removal of Chromium (VI) from Aqueous Solution Using Electro-Fenton Process. Environ. Processes 2015, 2, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Higgins, T.E.; Halloran, A.R.; Petura, J.C. Traditional and innovative treatment methods for Cr(VI) in soil. J. Soil Contam. 1997, 6, 767–797. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Pawar, R.R.; Xiong, Y.; Dabo, I.; Ohtani, B.; Kim, Y.; Song, J.T.; Ishihara, T.; Sasaki, K. Energy-resolved distribution of electron traps for O/S-doped carbon nitrides by reversed double-beam photoacoustic spectroscopy and the photocatalytic reduction of Cr(vi). Chem. Commun. 2020, 56, 3793–3796. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhou, X.; Fu, B.; Chen, Y. Catalytic wet peroxide oxidation of azo dye (Direct Blue 15) using solvothermally synthesized copper hydroxide nitrate as catalyst. J. Hazard. Mater. 2011, 187, 348–354. [Google Scholar] [CrossRef]
- Jia, J.; Wang, H.; Niu, H.; Chen, J.; Song, J.; Mao, C.; Zhang, S.; Gao, Y.; Chen, C. Highly selective adsorption of organic dyes containing sulphonic groups using Cu2(OH)3NO3 nanosheets. J. Nanopart. Res. 2016, 18, 260. [Google Scholar] [CrossRef]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Rajamathi, J.T.; Britto, S.; Rajamathi, M. Synthesis and anion exchange reactions of a layered copper-zinc hydroxy double salt, Cu1.6Zn0.4(OH)3(OAc)·H2O. J. Chem. Sci. 2005, 117, 629–633. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Sun, Y.; Yang, S.; Song, X.; Yang, Z. Hierarchical CuO nanoflowers: Water-required synthesis and their application in a nonenzymatic glucose biosensor. Phys. Chem. Chem. Phys. 2013, 15, 10904–10913. [Google Scholar] [CrossRef]
- Bourenane, N.; Hamlaoui, Y.; Remazeilles, C.; Pedraza, F. Effect of the pH of the electrolyte on the formation and on the corrosion properties of ceria based coating on carbon steel. Mater. Corros. 2019, 70, 110–119. [Google Scholar] [CrossRef]
- Wang, C.; Higgins, D.; Wang, F.; Li, D.; Liu, R.; Xia, G.; Li, N.; Li, Q.; Xu, H.; Wu, G. Controlled synthesis of micro/nanostructured CuO anodes for lithium-ion batteries. Nano Energy 2014, 9, 334–344. [Google Scholar] [CrossRef]
- Liang, W.; Zhu, L.; Li, W.; Liu, H. Facile fabrication of a flower-like CuO/Cu(OH)2 nanorod film with tunable wetting transition and excellent stability. RSC Adv. 2015, 5, 38100–38110. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yan, T.; Zhu, R.; Yan, L.; Pei, Z. Adsorption and photocatalytic reduction of aqueous Cr(VI) by Fe3O4-ZnAl-layered double hydroxide/TiO2 composites. J. Colloid Interface Sci. 2020, 562, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, Q.; Zhang, Y.; Yang, Z.; Zhu, A.; Dionysiou, D.D. Enhancement of the Cr(VI) adsorption and photocatalytic reduction activity of g-C3N4 by hydrothermal treatment in HNO3 aqueous solution. Appl. Catal. A Gen. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Ali, S.; Sriubas, M.; Varnagiris, S.; Tuckute, S.; Abdelnaby, M.A.; Kamel, B.M. Highly Efficient Visible Light Photodegradation of Cr(VI) Using Electrospun MWCNTs-Fe3O4@PES Nanofibers. Catalysts 2021, 11, 868. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Chondrodimou, I.; Bairamis, F.; Giannakas, A.; Konstantinou, I. Photocatalytic reduction of Cr(VI) by char/TiO2 composite photocatalyst: Optimization and modeling using the response surface methodology (RSM). Environ. Sci. Pollut. Res. 2017, 24, 1063–1072. [Google Scholar] [CrossRef]
- Laipan, M.; Zhu, R.; Zhu, J.; He, H. Visible light assisted Fenton-like degradation of Orange II on Ni3Fe/Fe3O4 magnetic catalyst prepared from spent FeNi layered double hydroxide. J. Mol. Catal. A Chem. 2016, 415, 9–16. [Google Scholar] [CrossRef]
- Costa, M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Chuaicham, C.; Pawar, R.R.; Sasaki, K.; Li, W.; Lee, A.F.; Wilson, K. Template free mild hydrothermal synthesis of core–shell Cu2O(Cu)@CuO visible light photocatalysts for N-acetyl-para-aminophenol degradation. J. Mater. Chem. A 2019, 7, 20767–20777. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Balijapalli, U.; Li, W.; Wilson, K.F.; Lee, A.; Sasaki, K. Surfactant- and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl. Catal. B Environ. 2021, 284, 119741. [Google Scholar] [CrossRef]
- Dvoranova, D.; Barbierikova, Z.; Brezova, V. Radical intermediates in photoinduced reactions on TiO2 (an EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuaicham, C.; Sekar, K.; Balakumar, V.; Zhang, L.; Trakulmututa, J.; Smith, S.M.; Sasaki, K. Fabrication of Hydrotalcite-like Copper Hydroxyl Salts as a Photocatalyst and Adsorbent for Hexavalent Chromium Removal. Minerals 2022, 12, 182. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020182
Chuaicham C, Sekar K, Balakumar V, Zhang L, Trakulmututa J, Smith SM, Sasaki K. Fabrication of Hydrotalcite-like Copper Hydroxyl Salts as a Photocatalyst and Adsorbent for Hexavalent Chromium Removal. Minerals. 2022; 12(2):182. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020182
Chicago/Turabian StyleChuaicham, Chitiphon, Karthikeyan Sekar, Vellaichamy Balakumar, Li Zhang, Jirawat Trakulmututa, Siwaporn Meejoo Smith, and Keiko Sasaki. 2022. "Fabrication of Hydrotalcite-like Copper Hydroxyl Salts as a Photocatalyst and Adsorbent for Hexavalent Chromium Removal" Minerals 12, no. 2: 182. https://0-doi-org.brum.beds.ac.uk/10.3390/min12020182