The Influence of Roasting Temperature on the Flotation Properties of Muscovite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Processes and Methods
3. Results
3.1. Structural Characterization of Roasted Muscovite
3.2. Surface Properties
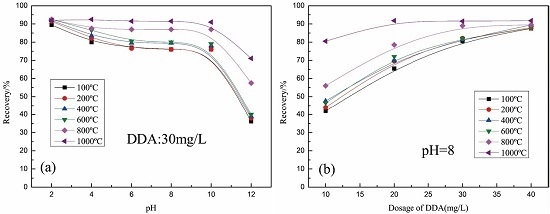
3.3. Flotation Properties
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114, 1–6. [Google Scholar] [CrossRef]
- Ye, P.H.; Wang, X.W.; Wang, M.Y.; Fan, Y.Y.; Xiang, X.Y. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching. Hydrometallurgy 2012, 117, 108–115. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Bian, Y.; Bao, S.X. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Bao, S.X.; Liu, T.; Bian, Y.; Liu, X.; Jiang, M.F. Separation factor of shaking table for vanadium pre-concentration from stone coal. Sep. Purif. Technol. 2013, 115, 92–99. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Liu, R.Q.; Gu, X.C. Flotation recovery of vanadium from low-grade stone coal. Trans. Nonferrous Met. Soc. 2014, 24, 1145–1151. [Google Scholar] [CrossRef]
- Wu, H.L.; Zhao, W.; Li, M.T.; Deng, G.Z.; Ge, H.H.; Wei, C. New craft study on enriching vanadium by means of priority coal flotation from high carbon stone-coal. J. Chin. Rare Earth Soc. 2008, 26, 530–533. [Google Scholar]
- Zhao, Y.L.; Zhang, Y.M.; Song, S.X.; Chen, T.J.; Bao, S.X. Behaviors of impurity elements Ca and Fe in vanadium-bearing stone coal during roasting and its control measure. Int. J. Miner. Process. 2016, 148, 100–104. [Google Scholar] [CrossRef]
- Chen, T.J.; Qiu, G.Z.; Zhu, D.Q. Oxidation mechanism of vanadium extraction in stone coal roasting with cyclic oxidation. Met. Mine. 2008, 6, 62–65. [Google Scholar]
- Chen, T.J.; Qiu, G.Z.; Zhu, D.Q. Valence variation and oxidation kinetics of vanadium during vanadium bearing stone coal roasting. Min. Metall. Eng. 2008, 3, 64–67. [Google Scholar]
- Nosrati, A.; Addai-Mensah, J.; Skinner, W. Muscovite clay mineral particle interactions in aqueous media. Powder Technol. 2012, 219, 228–238. [Google Scholar]
- Yan, L.J.; Masliyah, J.H.; Xu, Z.H. Interaction of divalent cations with basal planes and edge surfaces of phyllosilicate minerals: Muscovite and Talc. J. Colloid Interface Sci. 2013, 404, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.J.; Rutland, M.W.; Manev, E.; Claesson, P.M. Dodecylamine collector—PH effect on mica flotation and correlation with thin aqueous foam film and surface force measurements. Int. J. Miner. Process. 1996, 46, 245–262. [Google Scholar] [CrossRef]
- Sekulić, Ž.; Canić, N.; Bartulović, Z.; Daković, A. Application of different collectors in the flotation concentration of feldspar, mica and quartz sand. Miner. Eng. 2004, 17, 77–80. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.X.; Zhang, Z.G. The Infrared Spectroscopy of Minerals; Chongqing Publishing House: Chongqing, China, 1998. (In Chinese) [Google Scholar]
- Zhou, Z.J.; Yang, Z.Y.; Chen, D.Z. The thermal swellability of illite from Duchuantou in Zhejiang province. J. Miner. Petrol. 1996, 65, 7–12. [Google Scholar]
- Gridi-Bennadji, F.; Beneu, B.; Laval, J.P.; Blanchart, P. Structural transformations of muscovite at high temperature by X-ray and neutron diffraction. Appl. Clay. Sci. 2008, 38, 259–267. [Google Scholar] [CrossRef]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Xu, L.H.; Wu, H.Q.; Dong, F.Q.; Wang, L.; Wang, Z.; Xiao, J.H. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]






| Na2O | MgO | Al2O3 | SiO2 | K2O | Fe2O3 |
|---|---|---|---|---|---|
| 0.50 | 0.92 | 30.64 | 46.95 | 10.57 | 5.12 |
| Diffraction Peak Number | Crystal Face | d Value | |||||
|---|---|---|---|---|---|---|---|
| Raw Ore | 200 °C | 400 °C | 600 °C | 800 °C | 1000 °C | ||
| 1 | 002 | 10.0416 | 10.0398 | 10.0406 | 10.0408 | 10.0424 | 10.1312 |
| 2 | 004 | 5.0071 | 5.0064 | 5.0070 | 5.0020 | 5.0300 | 5.0569 |
| 3 | 006 | 3.3336 | 3.3312 | 3.3338 | 3.3312 | 3.3556 | 3.3654 |
| 4 | 114 | 3.2088 | 3.2088 | 3.2108 | 3.2087 | 3.2270 | 3.2382 |
| 5 | 025 | 2.9976 | 2.9976 | 2.9994 | 3.0012 | 2.9985 | 3.0355 |
| 6 | 115 | 2.8696 | 2.8680 | 2.8678 | 2.8713 | 2.8891 | 2.8952 |
| 7 | 008 | 2.4993 | 2.4980 | 2.4994 | 2.4979 | 2.5169 | 2.5225 |
| 8 | 029 | 1.9977 | 1.9969 | 1.9985 | 1.9961 | 2.0136 | 2.0171 |
| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O1s | Si2p | Al2p | Fe2p | K2p | N1s | C1s | O1s | Si2p | Al2p | Fe2p | K2p | |
| Raw Ore | 531.5 | 102.4 | 74.1 | 711.8 | 292.9 | - | 284.6 | - | - | - | - | - |
| 1000 °C | 531.3 | 102.3 | 73.9 | 711.6 | 293.0 | - | 284.6 | −0.2 | −0.1 | −0.2 | −0.2 | +0.1 |
| Raw Ore + DDA | 531.5 | 102.4 | 74.1 | 711.8 | 292.8 | 401.5 | 284.6 | +0.0 | +0.0 | +0.0 | +0.0 | −0.1 |
| 1000 °C + DDA | 531.3 | 102.3 | 74.0 | 711.6 | 293.0 | 401.2 | 284.6 | −0.2 | −0.1 | −0.1 | −0.2 | +0.1 |
| Sample | Relative Contents (%) | ||||||
|---|---|---|---|---|---|---|---|
| O1s | Si2p | Al2p | Fe2p | K2p | N1s | C1s | |
| Raw Ore | 54.8 | 18.3 | 14.5 | 1.5 | 4.5 | - | 6.5 |
| 1000 °C | 54.1 | 17.8 | 13.3 | 1.4 | 4.6 | - | 8.8 |
| Raw Ore + DDA | 51.0 | 17.6 | 13.8 | 1.4 | 4.1 | 2.5 | 11.0 |
| 1000 °C + DDA | 52.1 | 17.2 | 13.4 | 1.4 | 4.3 | 1.0 | 10.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Zhang, Y.; Bao, S. The Influence of Roasting Temperature on the Flotation Properties of Muscovite. Minerals 2016, 6, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/min6020053
Tang J, Zhang Y, Bao S. The Influence of Roasting Temperature on the Flotation Properties of Muscovite. Minerals. 2016; 6(2):53. https://0-doi-org.brum.beds.ac.uk/10.3390/min6020053
Chicago/Turabian StyleTang, Jiayan, Yimin Zhang, and Shenxu Bao. 2016. "The Influence of Roasting Temperature on the Flotation Properties of Muscovite" Minerals 6, no. 2: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/min6020053