Atherosclerosis and Its Impact on the Outcomes of Patients with Deep Venous Thrombosis
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Study Sample
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
3.1. Comparison of DVT Patients with and without Additional PE: Isolated DVT (without Concomitant PE) Is Accompanied by Unfavorable Cardiovascular Profile
3.2. Comparison of Patients with Isolated Deep Venous Thrombosis (without Concomitant Pulmonary Embolism) vs. Patients with Isolated Pulmonary Embolism (without Concomitant Deep Venous Thrombosis)
3.3. Baseline Comparison between DVT Patients with and without Symptomatic Atherosclerosis
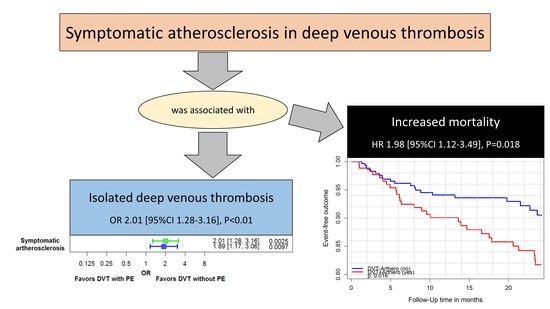
3.4. Impact of Symptomatic Atherosclerosis on Outcomes in Patients with Deep Venous Thrombosis
4. Discussion
- (i)
- Isolated DVT (without concomitant PE) was accompanied by a substantially higher prevalence of symptomatic atherosclerosis and related diseases in comparison to DVT patients with PE;
- (ii)
- Diabetes mellitus, a key player in the development of atherosclerosis, might also have a key role in DVT patients without PE;
- (iii)
- The prevalence of PAD was substantially higher in patients with isolated DVT (without concomitant PE) in comparison to isolated PE (without concomitant DVT);
- (iv)
- Obesity seems to be an independent risk factor for the development of PE in DVT patients;
- (v)
- Isolated PE (without concomitant DVT) was associated with old age, chronic lung diseases and AF;
- (vi)
- Symptomatic atherosclerosis affected mortality and thromboembolic arterial and venous events in DVT patients under VKA treatment during the 2-year and 3-year follow-up period.
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| CI | Confidence interval |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CLD | Chronic lung diseases |
| COPD | Chronic obstructive pulmonary disease |
| CS | Coagulation service |
| CVRF | Cardiovascular risk factors |
| DVT | Deep vein thrombosis |
| FH | Family history |
| HF | Congestive heart failure |
| MI | Myocardial infarction |
| OAC | Oral anticoagulant treatment |
| OR | Odds ratio |
| PAD | Peripheral artery disease |
| PE | Pulmonary embolism |
| RMC | Regular medical care |
| VKA | Vitamin K antagonist treatment |
| VTE | Venous thromboembolism |
References
- Oger, E. Incidence of venous thromboembolism: A community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb. Haemost. 2000, 83, 657–660. [Google Scholar] [PubMed]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapson, V.F. Acute Pulmonary Embolism. N. Engl. J. Med. 2008, 358, 1037–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinides, S.V.; Meyer, G. The 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2019, 40, 3453–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, J.A.; Solano, R.; Iglesias, N.T.; Ruiz-Giménez, N.; Fernández-Capitán, C.; Valero, B.; Tiberio, G.; Bura-Riviere, A.; Monreal, M. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thromb. Res. 2013, 132, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.-P.; Münzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.D.; Goodin, A.J.; Lip, G.Y.H.; Adams, V.R. Risk Stratification for Bleeding Complications in Patients with Venous Thromboembolism: Application of the HAS-BLED Bleeding Score During the First 6 Months of Anticoagulant Treatment. J. Am. Heart Assoc. 2018, 7, e007901. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Barco, S.; Lankeit, M.; Meyer, G. Management of Pulmonary Embolism: An Update. J. Am. Coll. Cardiol. 2016, 67, 976–990. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Van Der Heijden, J.F.; Hutten, B.A.; Büller, H.R.; Prins, M.H. Vitamin K antagonists or low-molecular-weight heparin for the long term treatment of symptomatic venous thromboembolism. Cochrane Database Syst. Rev. 2002, CD002001. [Google Scholar] [CrossRef]
- Hutten, B.A.; Prins, M.H. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst. Rev. 2014, 2014, CD001367. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Martinez, I.; Emmerich, J. Venous Thromboembolism: Risk factors for recurrence. Arter. Thromb. Vasc. Biol. 2009, 29, 298–310. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Elliott, C.G. Acute Pulmonary Embolism: Part I: Epidemiology, pathophysiology, and diagnosis. Circulation 2003, 108, 2726–2729. [Google Scholar] [CrossRef] [Green Version]
- Kyrle, P.A.; Eichinger, S. Deep vein thrombosis. Lancet 2005, 365, 1163–1174. [Google Scholar] [CrossRef]
- Prandoni, P. Links between arterial and venous disease. J. Intern. Med. 2007, 262, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.D. The incidence of pulmonary embolism during deep vein thrombosis. Phlebol. J. Venous Dis. 2013, 28 (Suppl. 1), 29–33. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, K.; Hobohm, L.; Münzel, T.; Ostad, M.A. Impact of concomitant deep or superficial venous thrombosis of the legs on survival of patients with pulmonary embolism. Int. J. Cardiol. 2020, 315, 92–98. [Google Scholar] [CrossRef]
- Keller, K.; Prochaska, J.H.; Coldewey, M.; Gobel, S.; Ullmann, A.; Jünger, C.; Lamparter, H.; Ariza, L.; Bickel, C.; Lauterbach, M.; et al. History of deep vein thrombosis is a discriminator for concomitant atrial fibrillation in pulmonary embolism. Thromb. Res. 2015, 136, 899–906. [Google Scholar] [CrossRef]
- Sørensen, H.T.; Horvath-Puho, E.; Lash, T.L.; Christiansen, C.F.; Pesavento, R.; Pedersen, L.; Baron, J.A.; Prandoni, P. Heart Disease May Be a Risk Factor for Pulmonary Embolism without Peripheral Deep Venous Thrombosis. Circulation 2011, 124, 1435–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, T.; Hingorani, A.; Ascher, E.; Marks, N.; Shiferson, A.; Jung, D.; Jimenez, R.; Jacob, T. Pulmonary Embolism without Deep Venous Thrombosis. Ann. Vasc. Surg. 2012, 26, 973–976. [Google Scholar] [CrossRef]
- Prandoni, P. Venous and arterial thrombosis: Two aspects of the same disease? Eur. J. Intern. Med. 2009, 20, 660–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.A.; Prins, M.H.; Girolami, A. An Association between Atherosclerosis and Venous Thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, H.T.; Horvath-Puho, E.; Pedersen, L.; Baron, J.A.; Prandoni, P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: A 20-year cohort study. Lancet 2007, 370, 1773–1779. [Google Scholar] [CrossRef]
- Libertiny, G.; Hands, L. Deep venous thrombosis in peripheral vascular disease. Br. J. Surg. 1999, 86, 907–910. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Münzel, T.; Lankeit, M.; Konstantinides, S.; Ostad, M.A. Impact of Systemic Atherosclerosis on Clinical Characteristics and Short-Term Outcomes in Patients with Deep Venous Thrombosis or Thrombophlebitis. Am. J. Med. Sci. 2021, 363, 232–241. [Google Scholar] [CrossRef]
- Rafi, S.; van Doormaal, F.; van Lienden, K.; Kamphuisen, P.; Gerdes, V. Venous thrombo-embolism and aortic calcifications; more evidence on the link between venous and arterial thrombosis. Thromb. Res. 2009, 124, 381–382. [Google Scholar] [CrossRef]
- Prandoni, P.; Ciammaichella, M.; Mumoli, N.; Zanatta, N.; Visonà, A.; Avruscio, G.; Camporese, G.; Bucherini, E.; Bova, C.; Imberti, D.; et al. An association between residual vein thrombosis and subclinical atherosclerosis: Cross-sectional study. Thromb. Res. 2017, 157, 16–19. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Münzel, T.; Ostad, M.A. Impact of symptomatic atherosclerosis in patients with pulmonary embolism. Int. J. Cardiol. 2019, 278, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Coldewey, M.; Göbel, S.; Keller, K.; Hendelmeier, M.; Konstantinides, S.; Münzel, T.; Wild, P.S.; for the thrombEVAL Study Group. Evaluation of oral anticoagulation therapy: Rationale and design of the thrombEVAL study programme. Eur. J. Prev. Cardiol. 2014, 22, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Göbel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Jünger, C.; Al-Bayati, Z.; Baer, C.; Walter, U.; et al. Quality of oral anticoagulation with phenprocoumon in regular medical care and its potential for improvement in a telemedicine-based coagulation service—Results from the prospective, multi-center, observational cohort study thrombEVAL. BMC Med. 2015, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michal, M.; Prochaska, J.H.; Ullmann, A.; Keller, K.; Göbel, S.; Coldewey, M.; Münzel, T.; Wiltink, J.; Beutel, M.E.; Wild, P.S. Relevance of depression for anticoagulation management in a routine medical care setting: Results from the ThrombEVAL study program. J. Thromb. Haemost. 2014, 12, 2024–2033. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.; Göbel, S.; Cate, V.T.; Panova-Noeva, M.; Eggebrecht, L.; Nagler, M.; Coldewey, M.; Foebel, M.; Bickel, C.; Lauterbach, M.; et al. Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study. J. Clin. Med. 2020, 9, 3281. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hansen-Krone, I.J.; Enga, K.F.; Njølstad, I.; Hansen, J.-B.; Braekkan, S.K. Heart healthy diet and risk of myocardial infarction and venous thromboembolism. Thromb. Haemost. 2012, 108, 554–560. [Google Scholar] [CrossRef]
- Mili, F.D.; Hooper, W.C.; Lally, C.; Austin, H. Family History of Myocardial Infarction Is a Risk Factor for Venous Thromboembolism Among Whites but Not Among Blacks. Clin. Appl. Thromb. Hemost. 2013, 19, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Zöller, B.; Li, X.; Sundquist, J.; Sundquist, K. Venous thromboembolism does not share strong familial susceptibility with coronary heart disease: A nationwide family study in Sweden. Eur. Heart J. 2011, 32, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Branchi, A.; Chantarangkul, V.; Clerici, M.; Merati, G.; Artoni, A.; Mannucci, P.M. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J. Thromb. Thrombolysis 2010, 31, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Zhu, F.; Du, D.; Pilgram, T.K.; Sicard, G.A.; Bae, K.T. Coronary artery calcification and risk factors for atherosclerosis in patients with venous thromboembolism. Atherosclerosis 2005, 183, 169–174. [Google Scholar] [CrossRef]
- Lowe, G.D.O. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008, 140, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z. Risk Factors for Venous Thromboembolism. J. Am. Coll. Cardiol. 2010, 56, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ageno, W.; Becattini, C.; Brighton, T.; Selby, R.; Kamphuisen, P.W. Cardiovascular Risk Factors and Venous Thromboembolism: A meta-analysis. Circulation 2008, 117, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandoni, P.; Prandoni, P. Venous thromboembolism and atherosclerosis: Is there a link? J. Thromb. Haemost. 2007, 5 (Suppl. 1), 270–275. [Google Scholar] [CrossRef]
- Green, D. Risk of future arterial cardiovascular events in patients with idiopathic venous thromboembolism. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Alenghat, F.J. The Prevalence of Atherosclerosis in Those with Inflammatory Connective Tissue Disease by Race, Age and Traditional Risk Factors. Sci. Rep. 2016, 6, 20303. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Kabrhel, C.; Varraso, R.; Goldhaber, S.Z.; Rimm, E.B.; Camargo, C.A. Prospective Study of BMI and the Risk of Pulmonary Embolism in Women. Obesity 2009, 17, 2040–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; De Staercke, C.; Hooper, W.C. The effects of obesity on venous thromboembolism: A review. Open J. Prev. Med. 2012, 2, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosson, J.L.; Sevestre, M.A.; Labarere, J.; Constans, J.; Quere, I.; Pernod, G. Recurrence and Mortality of DVT-Associated PE Is Greater Than Isolated PE Alone: Results of the 7532-Patients Prospective OPTIMEV Cohort Study. Blood 2007, 110, 700. [Google Scholar] [CrossRef]
- Keller, K.; Beule, J.; Balzer, J.O.; Dippold, W. D-Dimer and thrombus burden in acute pulmonary embolism. Am. J. Emerg. Med. 2018, 36, 1613–1618. [Google Scholar] [CrossRef]
- Becattini, C.; Cohen, A.T.; Agnelli, G.; Howard, L.; Castejón, B.; Trujillo-Santos, J.; Monreal, M.; Perrier, A.; Yusen, R.D.; Jiménez, D. Risk Stratification of Patients with Acute Symptomatic Pulmonary Embolism Based on Presence or Absence of Lower Extremity DVT: Systematic Review and Meta-analysis. Chest 2016, 149, 192–200. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar] [CrossRef] [Green Version]
- Lassila, R. Role and management of coagulation disorders in peripheral arterial disease. Scand. J. Surg. 2012, 101, 94–99. [Google Scholar] [CrossRef]
- Folsom, A.R.; Wu, K.K.; Shahar, E.; Davis, C.E. Association of hemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Arterioscler. Thromb. J. Vasc. Biol. 1993, 13, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Grainge, M.J.; West, J.; Card, T.R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Fox, E.A.; Kahn, S.R. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb. Haemost. 2005, 94, 362–365. [Google Scholar] [CrossRef]
- Chung, W.-S.; Lin, C.-L. Increased risks of venous thromboembolism in patients with psoriasis. A Nationwide Cohort Study. Thromb. Haemost. 2017, 117, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; McGill, N.K.; Shin, D.B.; Takeshita, J.; Löve, J.; Noe, M.H.; Fuxench, Z.C.C.; Choi, H.K.; Mehta, N.N.; Gelfand, J. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A general population-based cohort study. Eur. Heart J. 2018, 39, 3608–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodigiani, C.; Ferrazzi, P.; Di Micco, P.; Librè, L.; Genovese, S.; Quaglia, I.; Rota, L.L. Is there a relationship between factor V Leiden and type 2 diabetes? J. Transl. Med. 2009, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, D.M.; Delate, T.; Clark, N.P.; Martell, C.; Tran, T.; Crowther, M.A.; Garcia, D.A.; Ageno, W.; Hylek, E.M. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood 2009, 114, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Petrauskiene, V.; Falk, M.; Waernbaum, I.; Norberg, M.; Eriksson, J.W. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia 2005, 48, 1017–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, G.; Goldhaber, S.Z.; Kroll, A.; Goldberg, R.J.; Emery, C.; Spencer, F.A. Venous Thromboembolism in Patients with Diabetes Mellitus. Am. J. Med. 2012, 125, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Franchini, M.; Targher, G. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 502–512. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- Davies, M.J.; Thomas, A. Thrombosis and Acute Coronary-Artery Lesions in Sudden Cardiac Ischemic Death. N. Engl. J. Med. 1984, 310, 1137–1140. [Google Scholar] [CrossRef]
- Rauch, U.; Osende, J.I.; Fuster, V.; Badimon, J.J.; Fayad, Z.; Chesebro, J.H. Thrombus formation on atherosclerotic plaques: Pathogenesis and clinical consequences. Ann. Intern. Med. 2001, 134, 224–238. [Google Scholar] [CrossRef]
- Falk, E.; Thuesen, L. Pathology of coronary microembolisation and no reflow. Heart 2003, 89, 983–985. [Google Scholar] [CrossRef] [PubMed]


| DVT without PE (n = 305) | DVT with PE (n = 204) | p-Value | |
|---|---|---|---|
| Age (years) | 70.0 (57.0/78.3) | 70.0 (54.0/77.0) | 0.49 |
| Sex (Male) | 145 (47.5%) | 100 (49.0%) | 0.79 |
| Classical cardiovascular risk factors | |||
| Obesity * | 96 (31.5%) | 77 (37.7%) | 0.15 |
| Diabetes mellitus | 86 (28.2%) | 33 (16.3%) | <0.01 |
| Arterial hypertension | 205 (67.2%) | 124 (60.8%) | 0.16 |
| Dyslipidemia | 134 (43.9%) | 84 (41.2%) | 0.58 |
| Family history of myocardial infarction or stroke | 126 (41.3%) | 62 (30.4%) | 0.02 |
| Smoking (ex- or current smoker) | 141 (46.2%) | 84 (41.2%) | 0.28 |
| Co-morbidities | |||
| Myocardial infarction | 59 (19.4%) | 24 (11.8%) | 0.03 |
| Coronary heart disease | 94 (32.3%) | 35 (17.8%) | <0.001 |
| Congestive heart failure | 87 (28.9%) | 37 (18.2%) | <0.01 |
| Atrial fibrillation | 121 (40.1%) | 47 (23.0%) | <0.0001 |
| Peripheral artery disease | 63 (21.3%) | 22 (10.9%) | <0.01 |
| Stroke | 45 (14.8%) | 21 (10.3%) | 0.18 |
| Chronic lung disease | 59 (19.5%) | 45 (22.3%) | 0.05 |
| Chronic kidney disease | 60 (19.7%) | 39 (19.2%) | 0.91 |
| Cancer | 62 (21.0%) | 38 (18.8%) | 0.57 |
| Depression | 32 (10.5%) | 21 (10.3%) | 1.00 |
| Symptomatic atherosclerosis † | 127 (42.9%) | 52 (26.4%) | <0.001 |
| Charlson comorbidity index § | 5.59 ± 3.10 | 4.93 ± 3.05 | 0.02 |
| Variable | DVT without Symptomatic Atherosclerosis (n = 314) | DVT with Symptomatic Atherosclerosis (n = 179) | p-Value |
|---|---|---|---|
| Age (years) | 63.0 (48.0–75.0) | 74.0 (65.0–80.0) | <0.0001 |
| Sex (Men) | 138 (43.9%) | 101 (56.4%) | 0.0087 |
| Classical cardiovascular risk factors | |||
| Obesity * | 95 (30.3%) | 71 (39.7%) | 0.038 |
| Diabetes mellitus | 47 (15.0%) | 67 (37.4%) | <0.0001 |
| Arterial Hypertension | 173 (55.1%) | 146 (81.6%) | <0.0001 |
| Dyslipidemia | 98 (31.2%) | 115 (64.2%) | <0.0001 |
| Family history of myocardial infarction or stroke | 97 (30.9%) | 89 (49.7%) | <0.0001 |
| Smoking (ex- or current smoker) | 118 (37.6%) | 98 (54.7%) | 0.00024 |
| Co-morbidities | |||
| Heart failure | 45 (14.4%) | 73 (41.2%) | <0.0001 |
| Atrial fibrillation | 73 (23.2%) | 86 (48.3%) | <0.0001 |
| Stroke | 38 (12.1%) | 26 (14.5%) | 0.49 |
| Chronic obstructive pulmonary disease | 55 (17.5%) | 47 (26.4%) | 0.021 |
| Chronic kidney disease | 42 (13.4%) | 54 (30.2%) | <0.0001 |
| Cancer | 62 (20.3%) | 34 (19.3%) | 0.81 |
| Depression | 33 (10.5%) | 16 (8.9%) | 0.64 |
| Charlson comorbidity index | 4.00 (2.00–6.00) | 7.00 (5.00–8.00) | <0.0001 |
| Univariable Analysis for 2-Year Follow-Up | Univariable Analysis for 3-Year Follow-Up | |||
|---|---|---|---|---|
| Outcomes | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| All-cause mortality | 1.98 (1.12–3.49) | 0.018 | 2.34 (1.41–3.88) | 0.0010 |
| Major or clinically relevant bleeding | 1.86 (0.96–3.63) | 0.067 | 1.78 (1.00–3.17) | 0.051 |
| Hospitalizations | 1.64 (1.21–2.21) | 0.0012 | 1.70 (1.28–2.26) | 0.00023 |
| Primary long-term outcome | 1.99 (1.31–3.04) | 0.0013 | 2.00 (1.37–2.90) | 0.00030 |
| Thromboembolic arterial and venous events | 2.13 (0.95–4.81) | 0.068 | 2.36 (1.15–4.83) | 0.0019 |
| Recurrent venous thromboembolism | 1.99 (0.36–10.88) | 0.43 | 2.37 (0.69–8.13) | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, K.; Prochaska, J.H.; Coldewey, M.; Göbel, S.; Schmitt, V.H.; Hahad, O.; Ullmann, A.; Nagler, M.; Lamparter, H.; Espinola-Klein, C.; et al. Atherosclerosis and Its Impact on the Outcomes of Patients with Deep Venous Thrombosis. Life 2022, 12, 734. https://0-doi-org.brum.beds.ac.uk/10.3390/life12050734
Keller K, Prochaska JH, Coldewey M, Göbel S, Schmitt VH, Hahad O, Ullmann A, Nagler M, Lamparter H, Espinola-Klein C, et al. Atherosclerosis and Its Impact on the Outcomes of Patients with Deep Venous Thrombosis. Life. 2022; 12(5):734. https://0-doi-org.brum.beds.ac.uk/10.3390/life12050734
Chicago/Turabian StyleKeller, Karsten, Jürgen H. Prochaska, Meike Coldewey, Sebastian Göbel, Volker H. Schmitt, Omar Hahad, Alexander Ullmann, Markus Nagler, Heidrun Lamparter, Christine Espinola-Klein, and et al. 2022. "Atherosclerosis and Its Impact on the Outcomes of Patients with Deep Venous Thrombosis" Life 12, no. 5: 734. https://0-doi-org.brum.beds.ac.uk/10.3390/life12050734