The Added Value of [18F]FDG PET/CT in the Management of Invasive Fungal Infections
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demography and IFI Characteristics
3.2. [18F]FDG PET/CT Studies
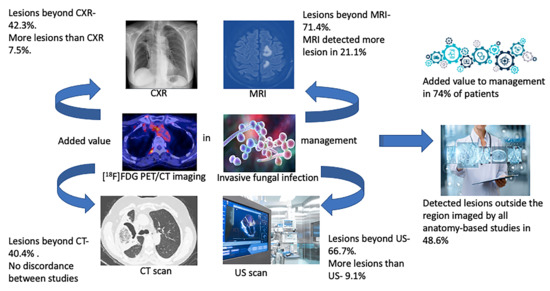
3.3. Anatomy-Based Studies
3.4. Discordance between [18F]FDG PET/CT and Anatomy-Based Imaging
3.5. Added Value Due to the Metabolic Assessment of Lesions by Patient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi (Basel) 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, A.O.; Sathekge, M.M.; Dierckx, R.A.; Glaudemans, A.W. Imaging fungal infections in children. Clin. Transl. Imaging 2016, 4, 57–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbach, W.J. Epidemiology of invasive fungal infections in neonates and children. Clin. Microbiol. Infect. 2010, 16, 1321–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlowski, H.L.P.; McWilliams, S.; Mellnick, V.M.; Bhalla, S.; Lubner, M.G.; Pickhardt, P.J.; Menias, C.O. Imaging Spectrum of Invasive Fungal and Fungal-like Infections. Radiographics 2017, 37, 1119–1134. [Google Scholar] [CrossRef]
- Ankrah, A.O.; Klein, H.C.; Span, L.F.R.; de Vries, E.F.J.; Dierckx, R.A.J.O.; Sathekge, M.M.; Glaudemans, A.W.J.M. The Role of PET in Monitoring Therapy in Fungal Infections. Curr. Pharm. Des. 2018, 24, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Leroy-Freschini, B.; Treglia, G.; Argemi, X.; Bund, C.; Kessler, R.; Herbrecht, R.; Imperiale, A. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM 2018, 111, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, S.; Patel, C.N.; Scarsbrook, A.F.; Chowdhury, F.U. FDG PET/CT in infection and inflammation--current and emerging clinical applications. Clin. Radiol. 2015, 70, 787–800. [Google Scholar] [CrossRef]
- Katragkou, A.; Fisher, B.T.; Groll, A.H.; Roilides, E.; Walsh, T.J. Diagnostic Imaging and Invasive Fungal Diseases in Children. J. Pediatric. Infect. Dis. Soc. 2017, 6, S22–S31. [Google Scholar] [CrossRef]
- Deutsch, P.G.; Whittaker, J.; Prasad, S. Invasive and Non-Invasive Fungal Rhinosinusitis-A Review and Update of the Evidence. Medicina (Kaunas) 2019, 55, 319. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, C.; Wang, X.; Zang, H.; Zhou, B. Clinical Features of Chronic Invasive Fungal Rhinosinusitis in 16 Cases. Ear Nose Throat J. 2020, 99, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Ankrah, A.O.; Span, L.F.R.; Klein, H.C.; de Jong, P.A.; Dierckx, R.A.J.O.; Kwee, T.C.; Sathekge, M.M.; Glaudemans, A.W.J.M. Role of FDG PET/CT in monitoring treatment response in patients with invasive fungal infections. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 174–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, A.P.; Thursky, K.A.; Worth, L.J.; Drummond, E.; Hogg, A.; Hicks, R.J.; Slavin, M.A. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: A retrospective comparison to conventional CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Macapinlac, H.A.; Kontoyiannis, D.P. The use of 18F-fluorodeoxyglucose positron emission tomography for the diagnosis and management of invasive mould infections. Med. Mycol. 2008, 46, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Hot, A.; Maunoury, C.; Poiree, S.; Lanternier, F.; Viard, J.P.; Loulergue, P.; Coignard, H.; Bougnoux, M.E.; Suarez, F.; Rubio, M.T.; et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin. Microbiol. Infect. 2011, 17, 409–417. [Google Scholar] [CrossRef] [Green Version]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Ankrah, A.O.; Lawal, I.; Vorster, M. Monitoring Response to Therapy. Semin. Nucl. Med. 2018, 48, 166–181. [Google Scholar] [CrossRef] [Green Version]
- Gariani, J.; Westerland, O.; Natas, S.; Verma, H.; Cook, G.; Goh, V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit. Rev. Oncol. Hematol. 2018, 124, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Self, W.H.; Courtney, D.M.; McNaughton, C.D.; Wunderink, R.G.; Kline, J.A. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: Implications for diagnosing pneumonia. Am. J. Emerg. Med. 2013, 31, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Ruhnke, M.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hamprecht, A.; Heinz, W.; Heussel, C.P.; Horger, M.; Kurzai, O.; Karthaus, M.; et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 2018, 61, 796–813. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Lin, X.; Wang, S.; Shen, Y.; Meng, Z.; Jia, Q.; Tan, J. Combination of positron emission tomography/computed tomography and chest thin-layer high-resolution computed tomography for evaluation of pulmonary nodules: Correlation with imaging features, maximum standardized uptake value, and pathology. Medicine (Baltimore). 2018, 97, e11640. [Google Scholar] [CrossRef] [PubMed]
- Hod, N.; Anconina, R.; Levin, D.; Tiktinsky, E.; Ezroh Kazap, D.; Levi, I.; Zektser, M.; Stavi, V.; Sebbag, G.; Lantsberg, S. Detection of Disseminated Aspergillosis on FDG PET/CT in a Patient with Acute Lymphoblastic Leukemia. Isr. Med. Assoc. J. 2018, 20, 720–721. [Google Scholar] [PubMed]
- Rammaert, B.; Desjardins, A.; Lortholary, O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses. 2012, 55, e74–e84. [Google Scholar] [CrossRef]
- Rossetti, F.; Brawner, D.L.; Bowden, R.; Meyer, W.G.; Schoch, H.G.; Fisher, L.; Myerson, D.; Hackman, R.C.; Shulman, H.M.; Sale, G.E.; et al. Fungal liver infection in marrow transplant recipients: Prevalence at autopsy, predisposing factors, and clinical features. Clin. Infect. Dis. 1995, 20, 801–811. [Google Scholar] [CrossRef]
- De La Cruz, O.; Silveira, F.P. Respiratory Fungal Infections in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin. Chest. Med. 2017, 38, 727–739. [Google Scholar] [CrossRef]
- Glaudemans, A.W.; de Vries, E.F.; Galli, F.; Dierckx, R.A.; Slart, R.H.; Signore, A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [Green Version]




| Number (%) (n = 73) | |
|---|---|
| Type of IFI | |
| Aspergillosis | 48 (66%) |
| Candidiasis | 21 (29%) |
| Other | 4 (5%) |
| Crytococcus neoformans Hormografiella aspergillata Pleurostomorpha richardsiae Alternaria alternata | 1 |
| 1 | |
| 1 | |
| 1 | |
| Classification of IFI | |
| Patients with proven IFIs | 42 (58%) |
| Patients with probable IFIs | 19 (26%) |
| Patients with possible IFIs | 12 (16%) |
| Underlying predisposing factor | |
| Acute leukemia | 27 (37%) |
| Hematologic conditions excluding acute leukemia | 18 (25%) |
| Solid-organ transplantation | 10 (14%) |
| Invasive procedures | 4 (4%) |
| High-dose steroids | 4 (5%) |
| Intense chemotherapy | 3 (4%) |
| Lung cavitation | 2 (3%) |
| No clear underlying conditions | 5 (7%) |
| Indication | Number (%) (n = 73) |
|---|---|
| Monitor response to antifungal therapy | 32 (44%) |
| Stage infection | 18 (25%) |
| Unexplained fever or increasing infective markers | 10 (14%) |
| Evaluation for ASCT (Allogeneic stem cell transplantation) | 7 (10%) |
| Detect active disease in residual lesions | 6 (8%) |
| Anatomy-Based Studies for Each [18F]FDG PET/CT Scan Performed | [18F]FDG PET/CT Scans Performed | [18F]FDG PET/CT Scans That Were Concordant with All the Anatomy-Based Studies Performed (%) |
|---|---|---|
| 0 | 34 (21.9%) | n/A |
| 1 | 43 (27.7%) | 42 (97.6%) |
| 2 | 62 (40.0%) | 53 (85.5%) |
| 3 | 15 (9.7%) | 13 (86.6%) |
| 4 | 1 (0.1%) | 1 (100%) |
| Total | 155 (100%) | 109 of 121 (90.1%) |
| Anatomy-Based Study | [18F]FDG PET/CT Performed 1 (n, %) | Concordance with [18F]FDG PET/CT (n, %) | [18F]FDG PET/CT That Detected Lesions outside Region Imaged 2 (n, %) |
|---|---|---|---|
| Chest X-ray | 80 (37%) | 74 (92.5%) | 37 (42.3%) |
| HR CT and thoracic CT | 62 (29%) | 62 (100%) | 22 (35.5%) |
| Extra thoracic CT scan | 27 (13%) | 27 (100%) | 14 (51.8%) |
| MRI | 14 (6%) | 11 (78.6%) | 10 (71.4%) |
| Ultrasound imaging | 33 (15%) | 30 (90.9%) | 22 (66.7%) |
| Overall | 216 (100%) | 204 (94.4%) | 105 (48.6%) |
| Value Added | Number (%) |
|---|---|
| (Total number of patients) | 73 (100%) |
| Assessing response to therapy | 32 (44%) |
| Detected previously undiagnosed sites of IFIs | 5 (7%) |
| Unexplained fever leading to biopsy-guided diagnosis of IFI | 4 (5%) |
| Evaluation for ASCT | 7 (10%) |
| Metabolic activity of residual IFI lesions on anatomy-based imaging | 6 (8%) |
| Total patient added value for [18F]FDG PET/CT | 54 (74%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankrah, A.O.; Creemers-Schild, D.; de Keizer, B.; Klein, H.C.; Dierckx, R.A.J.O.; Kwee, T.C.; Span, L.F.R.; de Jong, P.A.; Sathekge, M.M.; Glaudemans, A.W.J.M. The Added Value of [18F]FDG PET/CT in the Management of Invasive Fungal Infections. Diagnostics 2021, 11, 137. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11010137
Ankrah AO, Creemers-Schild D, de Keizer B, Klein HC, Dierckx RAJO, Kwee TC, Span LFR, de Jong PA, Sathekge MM, Glaudemans AWJM. The Added Value of [18F]FDG PET/CT in the Management of Invasive Fungal Infections. Diagnostics. 2021; 11(1):137. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11010137
Chicago/Turabian StyleAnkrah, Alfred O., Dina Creemers-Schild, Bart de Keizer, Hans C. Klein, Rudi A. J. O. Dierckx, Thomas C. Kwee, Lambert F. R. Span, Pim A. de Jong, Mike M. Sathekge, and Andor W. J. M. Glaudemans. 2021. "The Added Value of [18F]FDG PET/CT in the Management of Invasive Fungal Infections" Diagnostics 11, no. 1: 137. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11010137